Regulation of GNRH production by estrogen and bone morphogenetic proteins in GT1-7 hypothalamic cells
- PMID: 19635757
- PMCID: PMC2768486
- DOI: 10.1677/JOE-09-0065
Regulation of GNRH production by estrogen and bone morphogenetic proteins in GT1-7 hypothalamic cells
Abstract
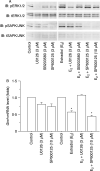
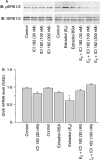
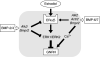
Recent studies have shown that bone morphogenetic proteins (BMPs) are important regulators in the pituitary-gonadal endocrine axis. We here investigated the effects of BMPs on GNRH production controlled by estrogen using murine GT1-7 hypothalamic neuron cells. GT1-7 cells expressed estrogen receptor alpha (ERalpha; ESR1 as listed in MGI Database), ERbeta (ESR2 as listed in MGI Database), BMP receptors, SMADs, and a binding protein follistatin. Treatment with BMP2 and BMP4 had no effect on Gnrh mRNA expression; however, BMP6 and BMP7 significantly increased Gnrh mRNA expression as well as GnRH production by GT1-7 cells. Notably, the reduction of Gnrh expression caused by estradiol (E(2)) was restored by cotreatment with BMP2 and BMP4, whereas it was not affected by BMP6 or BMP7. E(2) activated extracellular signal-regulated kinase (ERK) 1/2 and stress-activated protein kinase/c-Jun NH(2)-terminal kinase (SAPK/JNK) signaling but did not activate p38-mitogen-activated protein kinase (MAPK) signaling in GT1-7 cells. Inhibition of ERK1/ERK2 reversed the inhibitory effect of estrogen on Gnrh expression, whereas SAPK/JNK inhibition did not affect the E(2) actions. Expression levels of Eralpha and Erbeta were reduced by BMP2 and BMP4, but were increased by BMP6 and BMP7. Treatment with an ER antagonist inhibited the E(2) effects on Gnrh suppression including reduction of E(2)-induced ERK phosphorylation, suggesting the involvement of genomic ER actions in Gnrh suppression. BMP2 and BMP4 also suppressed estrogen-induced phosphorylation of ERK1/ERK2 and SAPK/JNK signaling, suggesting that BMP2 and BMP4 downregulate estrogen effects by attenuating ER-MAPK signaling. Considering that BMP6 and BMP7 increased the expression of alpha1E-subunit of R-type calcium channel (Cacna1e), which is critical for GNRH secretion, it is possible that BMP6 and BMP7 directly stimulate GNRH release by GT1-7 cells. Collectively, a newly uncovered interaction of BMPs and ER may be involved in controlling hypothalamic GNRH production and secretion via an autocrine/paracrine mechanism.
Figures






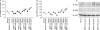
Similar articles
-
Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1-7 cells.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):8-15. doi: 10.1016/j.mce.2013.07.009. Epub 2013 Jul 20. Mol Cell Endocrinol. 2013. PMID: 23880664 Free PMC article.
-
Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation.J Endocrinol. 2008 Dec;199(3):445-55. doi: 10.1677/JOE-08-0226. Epub 2008 Sep 9. J Endocrinol. 2008. PMID: 18780779
-
Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons.Endocrinology. 1999 Nov;140(11):5045-53. doi: 10.1210/endo.140.11.7117. Endocrinology. 1999. PMID: 10537130
-
In vitro paradigms for the study of GnRH neuron function and estrogen effects.Ann N Y Acad Sci. 2003 Dec;1007:129-42. doi: 10.1196/annals.1286.013. Ann N Y Acad Sci. 2003. PMID: 14993047 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Unique bioactivities of bone morphogenetic proteins in regulation of reproductive endocrine functions.Reprod Med Biol. 2011 Apr 14;10(3):131-142. doi: 10.1007/s12522-011-0082-9. eCollection 2011 Sep. Reprod Med Biol. 2011. PMID: 29662354 Free PMC article. Review.
-
Interaction between gonadotropin-releasing hormone and bone morphogenetic protein-6 and -7 signaling in LβT2 gonadotrope cells.Mol Cell Endocrinol. 2012 Jan 2;348(1):147-54. doi: 10.1016/j.mce.2011.08.001. Epub 2011 Aug 9. Mol Cell Endocrinol. 2012. PMID: 21846488 Free PMC article.
-
Genome-wide association study applied to prolificacy in Santa Inês sheep.Trop Anim Health Prod. 2025 Apr 11;57(3):169. doi: 10.1007/s11250-025-04424-5. Trop Anim Health Prod. 2025. PMID: 40214842
-
GnRH agonist reduces estrogen receptor dimerization in GT1-7 cells: evidence for cross-talk between membrane-initiated estrogen and GnRH signaling.Mol Cell Endocrinol. 2015 Mar 15;404:67-74. doi: 10.1016/j.mce.2015.01.023. Epub 2015 Jan 22. Mol Cell Endocrinol. 2015. PMID: 25619861 Free PMC article.
-
Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1-7 cells.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):8-15. doi: 10.1016/j.mce.2013.07.009. Epub 2013 Jul 20. Mol Cell Endocrinol. 2013. PMID: 23880664 Free PMC article.
References
-
- Avola R, Spina-Purrello V, Gallo F, Morale MC, Marletta N, Costa A, Tirolo C, Testa N, Reale S, Marchetti B. Immortalized hypothalamic luteinizing hormone-releasing hormone (LHRH) neurons induce a functional switch in the growth factor responsiveness of astroglia: involvement of basic fibroblast growth factor. International Journal of Developmental Neuroscience. 2000;18:743–763. - PubMed
-
- Bowe J, Li XF, Sugden D, Katzenellenbogen JA, Katzenellenbogen BS, O'Byrne KT. The effects of the phytoestrogen, coumestrol, on gonadotropin-releasing hormone (GnRH) mRNA expression in GT1-7 GnRH neurones. Journal of Neuroendocrinology. 2003;15:105–108. - PubMed
-
- Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1). Biology of Reproduction. 2000;62:1710–1721. - PubMed
-
- Charles AC, Hales TG. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. Journal of Neurophysiology. 1995;73:56–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

