Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes
- PMID: 19635803
- PMCID: PMC2785336
- DOI: 10.1074/jbc.M109.009340
Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes
Abstract
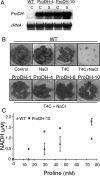
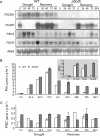
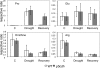
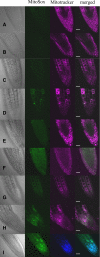
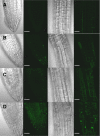
The two-step oxidation of proline in all eukaryotes is performed at the inner mitochondrial membrane by the consecutive action of proline dehydrogenase (ProDH) that produces Delta(1)-pyrroline-5-carboxylate (P5C) and P5C dehydrogenase (P5CDH) that oxidizes P5C to glutamate. This catabolic route is down-regulated in plants during osmotic stress, allowing free Pro accumulation. We show here that overexpression of MsProDH in tobacco and Arabidopsis or impairment of P5C oxidation in the Arabidopsis p5cdh mutant did not change the cellular Pro to P5C ratio under ambient and osmotic stress conditions, indicating that P5C excess was reduced to Pro in a mitochondrial-cytosolic cycle. This cycle, involving ProDH and P5C reductase, exists in animal cells and now demonstrated in plants. As a part of the cycle, Pro oxidation by the ProDH-FAD complex delivers electrons to the electron transport chain. Hyperactivity of the cycle, e.g. when an excess of exogenous l-Pro is provided, generates mitochondrial reactive oxygen species (ROS) by delivering electrons to O(2), as demonstrated by the mitochondria-specific MitoSox staining of superoxide ions. Lack of P5CDH activity led to higher ROS production under dark and light conditions in the presence of Pro excess, as well as rendered plants hypersensitive to heat stress. Balancing mitochondrial ROS production during increased Pro oxidation is therefore critical for avoiding Pro-related toxic effects. Hence, normal oxidation of P5C to Glu by P5CDH is key to prevent P5C-Pro intensive cycling and avoid ROS production from electron run-off.
Figures

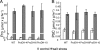
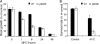
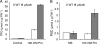
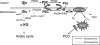
References
-
- Hu C. A., Donald S. P., Yu J., Lin W. W., Liu Z., Steel G., Obie C., Valle D., Phang J. M. (2007) Mol. Cell Biochem. 295, 85–92 - PubMed
-
- Hare P. D., Cress W. A. (1997) Plant Growth Regul. 21, 79–102
-
- Delauney A. J., Verma D. P. S. (1993) Plant J. 4, 215–223
-
- Chen T. H., Murata N. (2002) Curr. Opin. Plant Biol. 5, 250–257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

