p85 Associates with unphosphorylated PTEN and the PTEN-associated complex
- PMID: 19635806
- PMCID: PMC2747981
- DOI: 10.1128/MCB.01649-08
p85 Associates with unphosphorylated PTEN and the PTEN-associated complex
Abstract
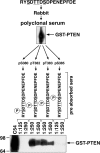
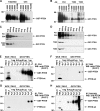
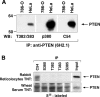
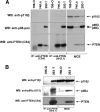
The lipid phosphatase PTEN functions as a tumor suppressor by dephosphorylating the D3 position of phosphoinositide-3,4,5-trisphosphate, thereby negatively regulating the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway. In mammalian cells, PTEN exists either as a monomer or as a part of a >600-kDa complex (the PTEN-associated complex [PAC]). Previous studies suggest that the antagonism of PI3K/AKT signaling by PTEN may be mediated by a nonphosphorylated form of the protein resident within the multiprotein complex. Here we show that PTEN associates with p85, the regulatory subunit of PI3K. Using newly generated antibodies, we demonstrate that this PTEN-p85 association involves the unphosphorylated form of PTEN engaged within the PAC and also includes the p110beta isoform of PI3K. The PTEN-p85 association is enhanced by trastuzumab treatment and linked to a decline in AKT phosphorylation in some ERBB2-amplified breast cancer cell lines. Together, these results suggest that integration of p85 into the PAC may provide a novel means of downregulating the PI3K/AKT pathway.
Figures
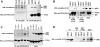

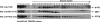

References
-
- Al-Khouri, A. M., Y. Ma, S. H. Togo, S. Williams, and T. Mustelin. 2005. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J. Biol. Chem. 280:35195-35202. - PubMed
-
- Anderson, C. W., J. W. Straus, and B. S. Dudock. 1983. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 101:635-644. - PubMed
-
- Barber, D. F., M. Alvarado-Kristensson, A. Gonzalez-Garcia, R. Pulido, and A. C. Carrera. 2006. PTEN regulation, a novel function for the p85 subunit of phosphoinositide 3-kinase. Sci. STKE 2006:pe49. - PubMed
-
- Baselga, J., L. Norton, J. Albanell, Y. M. Kim, and J. Mendelsohn. 1998. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 58:2825-2831. - PubMed
-
- Berns, K., H. M. Horlings, B. T. Hennessy, M. Madiredjo, E. M. Hijmans, K. Beelen, S. C. Linn, A. M. Gonzalez-Angulo, K. Stemke-Hale, M. Hauptmann, R. L. Beijersbergen, G. B. Mills, M. J. van de Vijver, and R. Bernards. 2007. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395-402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
