The vesicular acetylcholine transporter is required for neuromuscular development and function
- PMID: 19635813
- PMCID: PMC2747982
- DOI: 10.1128/MCB.00245-09
The vesicular acetylcholine transporter is required for neuromuscular development and function
Abstract
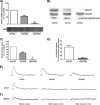
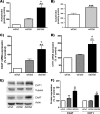
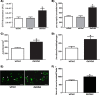
The vesicular acetylcholine (ACh) transporter (VAChT) mediates ACh storage by synaptic vesicles. However, the VAChT-independent release of ACh is believed to be important during development. Here we generated VAChT knockout mice and tested the physiological relevance of the VAChT-independent release of ACh. Homozygous VAChT knockout mice died shortly after birth, indicating that VAChT-mediated storage of ACh is essential for life. Indeed, synaptosomes obtained from brains of homozygous knockouts were incapable of releasing ACh in response to depolarization. Surprisingly, electrophysiological recordings at the skeletal-neuromuscular junction show that VAChT knockout mice present spontaneous miniature end-plate potentials with reduced amplitude and frequency, which are likely the result of a passive transport of ACh into synaptic vesicles. Interestingly, VAChT knockouts exhibit substantial increases in amounts of choline acetyltransferase, high-affinity choline transporter, and ACh. However, the development of the neuromuscular junction in these mice is severely affected. Mutant VAChT mice show increases in motoneuron and nerve terminal numbers. End plates are large, nerves exhibit abnormal sprouting, and muscle is necrotic. The abnormalities are similar to those of mice that cannot synthesize ACh due to a lack of choline acetyltransferase. Our results indicate that VAChT is essential to the normal development of motor neurons and the release of ACh.
Figures





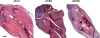
Similar articles
-
VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions.Skelet Muscle. 2016 Oct 5;6:31. doi: 10.1186/s13395-016-0105-7. eCollection 2016. Skelet Muscle. 2016. PMID: 27713817 Free PMC article.
-
Motoneuron-specific loss of VAChT mimics neuromuscular defects seen in congenital myasthenic syndrome.FEBS J. 2021 Sep;288(18):5331-5349. doi: 10.1111/febs.15825. Epub 2021 Apr 25. FEBS J. 2021. PMID: 33730374
-
Quantal release of acetylcholine in mice with reduced levels of the vesicular acetylcholine transporter.J Neurochem. 2010 May;113(4):943-51. doi: 10.1111/j.1471-4159.2010.06657.x. Epub 2010 Feb 25. J Neurochem. 2010. PMID: 20202084 Free PMC article.
-
Regulation of cholinergic activity by the vesicular acetylcholine transporter.Biochem J. 2013 Mar 1;450(2):265-74. doi: 10.1042/BJ20121662. Biochem J. 2013. PMID: 23410039 Review.
-
Microscopic kinetics and structure-function analysis in the vesicular acetylcholine transporter.Neurochem Int. 2002 Nov;41(5):285-9. doi: 10.1016/s0197-0186(02)00058-x. Neurochem Int. 2002. PMID: 12176068 Review.
Cited by
-
Cardiomyocyte-secreted acetylcholine is required for maintenance of homeostasis in the heart.FASEB J. 2013 Dec;27(12):5072-82. doi: 10.1096/fj.13-238279. Epub 2013 Sep 9. FASEB J. 2013. PMID: 24018063 Free PMC article.
-
Changes in the Gene Expression Profiles of the Inferior Colliculus Following Unilateral Cochlear Ablation in Adult Rats.Biochem Genet. 2021 Jun;59(3):731-750. doi: 10.1007/s10528-021-10034-1. Epub 2021 Jan 30. Biochem Genet. 2021. PMID: 33515340
-
Proenkephalin 119-159 in Heart Failure: From Pathophysiology to Clinical Implications.J Clin Med. 2025 Apr 13;14(8):2657. doi: 10.3390/jcm14082657. J Clin Med. 2025. PMID: 40283487 Free PMC article. Review.
-
Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits.Pharmacol Ther. 2021 Jun;222:107785. doi: 10.1016/j.pharmthera.2020.107785. Epub 2020 Dec 10. Pharmacol Ther. 2021. PMID: 33310157 Free PMC article. Review.
-
Striatal Acetylcholine Helps to Preserve Functional Outcomes in a Mouse Model of Stroke.ASN Neuro. 2020 Jan-Dec;12:1759091420961612. doi: 10.1177/1759091420961612. ASN Neuro. 2020. PMID: 32967452 Free PMC article.
References
-
- Alfonso, A., K. Grundahl, J. S. Duerr, H. P. Han, and J. B. Rand. 1993. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261:617-619. - PubMed
-
- Barbosa, J., Jr., A. D. Clarizia, M. V. Gomez, M. A. Romano-Silva, V. F. Prado, and M. A. Prado. 1997. Effect of protein kinase C activation on the release of [3H]acetylcholine in the presence of vesamicol. J. Neurochem. 69:2608-2611. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Brittain, R. T., G. P. Levy, and M. B. Tyers. 1969. The neuromuscular blocking action of 2-(4-phenylpiperidino)cyclohexanol (AH 5183). Eur. J. Pharmacol. 8:93-99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
