Electrical coupling synchronises spinal motoneuron activity during swimming in hatchling Xenopus tadpoles
- PMID: 19635820
- PMCID: PMC2766650
- DOI: 10.1113/jphysiol.2009.173468
Electrical coupling synchronises spinal motoneuron activity during swimming in hatchling Xenopus tadpoles
Abstract
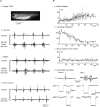
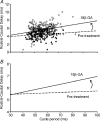
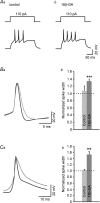
The role of electrical coupling between neurons in the swimming rhythm generator of Xenopus embryos has been studied using pharmacological blockade of gap junctions. A conspicuous effect of 18beta-glycyrrhetinic acid (18beta-GA) and carbenoxolone, which have been shown to block electrical coupling in this preparation, was to increase the duration of ventral root bursts throughout the spinal cord during swimming. The left-right coordination, the swimming frequency and the duration of swimming episodes were not affected by concentrations of 18beta-GA which significantly increased burst durations. However, the longitudinal coupling was affected such that 18beta-GA led to a significant correlation between rostrocaudal delays and cycle periods, which is usually only present in older larval animals. Patch clamp recordings from spinal motoneurons tested whether gap junction blockers affect the spike timing and/or firing pattern of motoneurons during fictive swimming. In the presence of 18beta-GA motoneurons continued to fire a single, but broader action potential in each cycle of swimming, and the timing of their spikes relative to the ventral root burst became more variable. 18beta-GA had no detectable effect on the resting membrane potential of motoneurons, but led to a significant increase in input resistance, consistent with the block of gap junctions. This effect did not result in increased firing during swimming, despite the fact that multiple spikes can occur in response to current injection. Applications of 18beta-GA at larval stage 42 had no discernible effect on locomotion. The results, which suggest that electrical coupling primarily functions to synchronize activity in synergistic motoneurons during embryo swimming, are discussed in the context of motor system development.
Figures
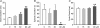



References
-
- Caveney S. The role of gap junctions in development. Annu Rev Physiol. 1985;47:319–335. - PubMed
-
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. - PubMed
-
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. - PubMed
-
- Dowling JE. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991;7:87–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

