Actin dynamics and Rho GTPases regulate the size and formation of parasitophorous vacuoles containing Coxiella burnetii
- PMID: 19635823
- PMCID: PMC2747940
- DOI: 10.1128/IAI.00301-09
Actin dynamics and Rho GTPases regulate the size and formation of parasitophorous vacuoles containing Coxiella burnetii
Abstract
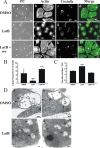
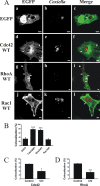
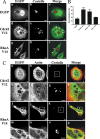
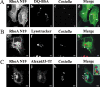
Q fever is a disease caused by Coxiella burnetii. In the host cell, this pathogen generates a large parasitophorous vacuole (PV) with lysosomal characteristics. Here we show that F-actin not only is recruited to but also is involved in the formation of the typical PV. Treatment of infected cells with F-actin-depolymerizing agents alters PV development. The small PVs formed in latrunculin B-treated cells were loaded with transferrin and Lysotracker and labeled with an antibody against cathepsin D, suggesting that latrunculin B did not affect vacuole cargo and its lysosomal characteristics. Nevertheless, the vacuoles were unable to fuse with latex bead phagosomes. It is known that actin dynamics are regulated by the Rho family GTPases. To assess the role of these GTPases in PV formation, infected cells were transfected with pEGFP expressing wild-type and mutant Rac1, Cdc42, and RhoA proteins. Rac1 did not show significant PV association. In contrast, PVs were decorated by both the wild types and constitutively active mutants of Cdc42 and RhoA. This association was inhibited by treatment of infected cells with chloramphenicol, suggesting a role for bacterial protein synthesis in the recruitment of these proteins. Interestingly, a decrease in vacuole size was observed in cells expressing dominant-negative RhoA; however, these small vacuoles accumulated transferrin, Lysotracker, and DQ-BSA. In summary, these results suggest that actin, likely modulated by the GTPases RhoA and Cdc42 and by bacterial proteins, is involved in the formation of the typical PV.
Figures
References
-
- Anes, E., M. P. Kuhnel, E. Bos, J. Moniz-Pereira, A. Habermann, and G. Griffiths. 2003. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat. Cell Biol. 5:793-802. - PubMed
-
- Anes, E., P. Peyron, L. Staali, L. Jordao, M. G. Gutierrez, H. Kress, M. Hagedorn, I. Maridonneau-Parini, M. A. Skinner, A. G. Wildeman, S. A. Kalamidas, M. Kuehnel, and G. Griffiths. 2006. Dynamic life and death interactions between Mycobacterium smegmatis and J774 macrophages. Cell. Microbiol. 8:939-960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

