Virulence and cellular interactions of Burkholderia multivorans in chronic granulomatous disease
- PMID: 19635825
- PMCID: PMC2747966
- DOI: 10.1128/IAI.00259-09
Virulence and cellular interactions of Burkholderia multivorans in chronic granulomatous disease
Abstract
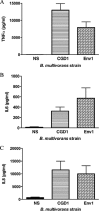
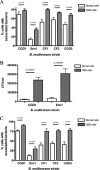
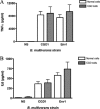
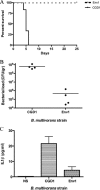
Chronic granulomatous disease (CGD) patients are susceptible to life-threatening infections by the Burkholderia cepacia complex. We used leukocytes from CGD and healthy donors and compared cell association, invasion, and cytokine induction by Burkholderia multivorans strains. A CGD isolate, CGD1, showed higher cell association than that of an environmental isolate, Env1, which correlated with cell entry. All B. multivorans strains associated significantly more with cells from CGD patients than with those from healthy donors. Similar findings were observed with another CGD pathogen, Serratia marcescens, but not with Escherichia coli. In a mouse model of CGD, strain CGD1 was virulent while Env1 was avirulent. B. multivorans organisms were found in the spleens of CGD1-infected mice at levels that were 1,000 times higher than those found in Env1-infected mice, which was coincident with higher levels of the proinflammatory cytokine interleukin-1beta. Taken together, these results may shed light on the unique susceptibility of CGD patients to specific pathogens.
Figures


Similar articles
-
Mutation of hmgA, encoding homogentisate 1,2-dioxygenase, is responsible for pyomelanin production but does not impact the virulence of Burkholderia cenocepacia in a chronic granulomatous disease mouse lung infection.Microbiol Spectr. 2024 Jul 2;12(7):e0041024. doi: 10.1128/spectrum.00410-24. Epub 2024 May 29. Microbiol Spectr. 2024. PMID: 38809005 Free PMC article.
-
Influence of neutrophil defects on Burkholderia cepacia complex pathogenesis.Front Cell Infect Microbiol. 2011 Nov 18;1:9. doi: 10.3389/fcimb.2011.00009. eCollection 2011. Front Cell Infect Microbiol. 2011. PMID: 22919575 Free PMC article. Review.
-
A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen.Infect Immun. 1999 Aug;67(8):4027-32. doi: 10.1128/IAI.67.8.4027-4032.1999. Infect Immun. 1999. PMID: 10417170 Free PMC article.
-
Virulence of Burkholderia cepacia complex strains in gp91phox-/- mice.Cell Microbiol. 2007 Dec;9(12):2817-25. doi: 10.1111/j.1462-5822.2007.00998.x. Epub 2007 Jul 11. Cell Microbiol. 2007. PMID: 17627623
-
Regulation of Virulence by Two-Component Systems in Pathogenic Burkholderia.Infect Immun. 2020 Jun 22;88(7):e00927-19. doi: 10.1128/IAI.00927-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32284365 Free PMC article. Review.
Cited by
-
Disparate properties of Burkholderia multivorans and Pseudomonas aeruginosa regarding outer membrane chemical permeabilization to the hydrophobic substances novobiocin and triclosan.PLoS One. 2023 Apr 25;18(4):e0284855. doi: 10.1371/journal.pone.0284855. eCollection 2023. PLoS One. 2023. PMID: 37098094 Free PMC article.
-
Immune Recognition of the Epidemic Cystic Fibrosis Pathogen Burkholderia dolosa.Infect Immun. 2017 May 23;85(6):e00765-16. doi: 10.1128/IAI.00765-16. Print 2017 Jun. Infect Immun. 2017. PMID: 28348057 Free PMC article.
-
Single amino acid substitution in homogentisate 1,2-dioxygenase is responsible for pigmentation in a subset of Burkholderia cepacia complex isolates.Environ Microbiol Rep. 2015 Apr;7(2):180-7. doi: 10.1111/1758-2229.12217. Epub 2014 Dec 17. Environ Microbiol Rep. 2015. PMID: 25294803 Free PMC article.
-
Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity.PLoS Pathog. 2013;9(7):e1003499. doi: 10.1371/journal.ppat.1003499. Epub 2013 Jul 18. PLoS Pathog. 2013. PMID: 23874203 Free PMC article.
-
Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex.J Infect Dis. 2010 Jun 15;201(12):1822-30. doi: 10.1086/652807. J Infect Dis. 2010. PMID: 20438352 Free PMC article.
References
-
- Baldwin, A., E. Mahenthiralingam, P. Drevinek, P. Vandamme, J. R. Govan, D. J. Waine, J. J. LiPuma, L. Chiarini, C. Dalmastri, D. A. Henry, D. P. Speert, D. Honeybourne, M. C. Maiden, and C. G. Dowson. 2007. Environmental Burkholderia cepacia complex isolates in human infections. Emerg. Infect. Dis. 13:458-461. - PMC - PubMed
-
- Bellinati-Pires, R., M. M. Salgado, I. P. Hypolito, A. S. Grumach, and M. M. Carneiro-Sampaio. 1995. Application of a fluorochrome-lysostaphin assay to the detection of phagocytic and bactericidal disturbances in human neutrophils and monocytes. J. Investig. Allergol. Clin. Immunol. 5:337-342. - PubMed
-
- Boyum, A. 1974. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens 4:269-274. - PubMed
-
- Bressler, A. M., K. S. Kaye, J. J. LiPuma, A. D. Alexander, C. M. Moore, L. B. Reller, and C. W. Woods. 2007. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without cystic fibrosis: a case control study. Infect. Control Hosp. Epidemiol. 28:951-958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

