Antigen display, T-cell activation, and immune evasion during acute and chronic ehrlichiosis
- PMID: 19635826
- PMCID: PMC2747950
- DOI: 10.1128/IAI.01433-08
Antigen display, T-cell activation, and immune evasion during acute and chronic ehrlichiosis
Abstract
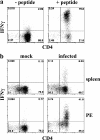
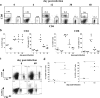
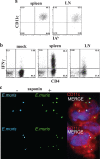
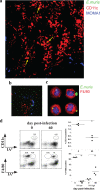
How spatial and temporal changes in major histocompatibility complex/peptide antigen presentation to CD4 T cells regulate CD4 T-cell responses during intracellular bacterial infections is relatively unexplored. We have shown that immunization with an ehrlichial outer membrane protein, OMP-19, protects mice against fatal ehrlichial challenge infection, and we identified a CD4 T-cell epitope (IA(b)/OMP-19(107-122)) that elicited CD4 T cells following either immunization or infection. Here, we have used an IA(b)/OMP-19(107-122)-specific T-cell line to monitor antigen display ex vivo during acute and chronic infection with Ehrlichia muris, a bacterium that establishes persistent infection in C57BL/6 mice. The display of IA(b)/OMP-19(107-122) by host antigen-presenting cells was detected by measuring intracellular gamma interferon (IFN-gamma) production by the T-cell line. After intravenous infection, antigen presentation was detected in the spleen, peritoneal exudate cells, and lymph nodes, although the kinetics of antigen display differed among the tissues. Antigen presentation and bacterial colonization were closely linked in each anatomical location, and there was a direct relationship between antigen display and CD4 T-cell effector function. Spleen and lymph node dendritic cells (DCs) were efficient presenters of IA(b)/OMP-19(107-122), demonstrating that DCs play an important role in ehrlichial infection and immunity. Chronic infection and antigen presentation occurred within the peritoneal cavity, even in the presence of highly activated CD4 T cells. These data indicated that the ehrlichiae maintain chronic infection not by inhibiting antigen presentation or T-cell activation but, in part, by avoiding signals mediated by activated T cells.
Figures


Similar articles
-
CD4 T-cell epitopes associated with protective immunity induced following vaccination of mice with an ehrlichial variable outer membrane protein.Infect Immun. 2007 Nov;75(11):5453-9. doi: 10.1128/IAI.00713-07. Epub 2007 Aug 13. Infect Immun. 2007. PMID: 17698576 Free PMC article.
-
Persistent infection contributes to heterologous protective immunity against fatal ehrlichiosis.Infect Immun. 2009 Dec;77(12):5682-9. doi: 10.1128/IAI.00720-09. Epub 2009 Oct 5. Infect Immun. 2009. PMID: 19805532 Free PMC article.
-
Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge.Infect Immun. 2008 May;76(5):1920-30. doi: 10.1128/IAI.01293-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285501 Free PMC article.
-
Dendritic cell subsets in T cell programming: location dictates function.Nat Rev Immunol. 2019 Feb;19(2):89-103. doi: 10.1038/s41577-018-0088-1. Nat Rev Immunol. 2019. PMID: 30464294 Free PMC article. Review.
-
Progress and obstacles in vaccine development for the ehrlichioses.Expert Rev Vaccines. 2010 Sep;9(9):1071-82. doi: 10.1586/erv.10.93. Expert Rev Vaccines. 2010. PMID: 20822349 Free PMC article. Review.
Cited by
-
IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection.J Immunol. 2011 Jan 15;186(2):1011-21. doi: 10.4049/jimmunol.1002836. Epub 2010 Dec 8. J Immunol. 2011. PMID: 21148037 Free PMC article.
-
TNF-α Contributes to Lymphoid Tissue Disorganization and Germinal Center B Cell Suppression during Intracellular Bacterial Infection.J Immunol. 2019 Nov 1;203(9):2415-2424. doi: 10.4049/jimmunol.1900484. Epub 2019 Sep 30. J Immunol. 2019. PMID: 31570507 Free PMC article.
-
Economic Game Theory to Model the Attenuation of Virulence of an Obligate Intracellular Bacterium.Front Cell Infect Microbiol. 2016 Aug 25;6:86. doi: 10.3389/fcimb.2016.00086. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27610355 Free PMC article.
-
T-bet+ B cells Dominate the Peritoneal Cavity B Cell Response during Murine Intracellular Bacterial Infection.J Immunol. 2022 Jun 15;208(12):2749-2760. doi: 10.4049/jimmunol.2101209. Epub 2022 Jun 3. J Immunol. 2022. PMID: 35867676 Free PMC article.
-
New insights into molecular Ehrlichia chaffeensis-host interactions.Microbes Infect. 2010 May;12(5):337-45. doi: 10.1016/j.micinf.2010.01.009. Epub 2010 Jan 29. Microbes Infect. 2010. PMID: 20116446 Free PMC article. Review.
References
-
- Agerer, F., S. Waeckerle, and C. R. Hauck. 2004. Microscopic quantification of bacterial invasion by a novel antibody-independent staining method. J. Microbiol. Methods 59:23-32. - PubMed
-
- Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3:619-626. - PubMed
-
- Balázs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341-352. - PubMed
-
- Bitsaktsis, C., and G. Winslow. 2006. Fatal recall responses mediated by CD8 T cells during intracellular bacteria infection. J. Immunol. 177:4644-4651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

