A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad
- PMID: 19635841
- PMCID: PMC2717649
- DOI: 10.1083/jcb.200902101
A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad
Abstract
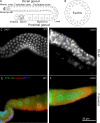
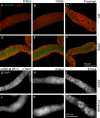
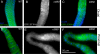
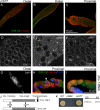
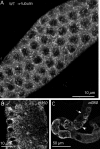
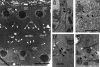
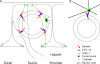
Changes in cellular microtubule organization often accompany developmental progression. In the Caenorhabditis elegans embryo, the centrosome, which is attached to the nucleus via ZYG-12, organizes the microtubule network. In this study, we investigate ZYG-12 function and microtubule organization before embryo formation in the gonad. Surprisingly, ZYG-12 is dispensable for centrosome attachment in the germline. However, ZYG-12-mediated recruitment of dynein to the nuclear envelope is required to maintain microtubule organization, membrane architecture, and nuclear positioning within the syncytial gonad. We examined gamma-tubulin localization and microtubule regrowth after depolymerization to identify sites of nucleation in germ cells. gamma-Tubulin localizes to the plasma membrane in addition to the centrosome, and regrowth initiates at both sites. Because we do not observe organized microtubules around zyg-12(ct350) mutant nuclei with attached centrosomes, we propose that gonad architecture, including membrane and nuclear positioning, is determined by microtubule nucleation at the plasma membrane combined with tension on the microtubules by dynein anchored at the nucleus by ZYG-12.
Figures


Similar articles
-
The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus.Cell. 2003 Dec 26;115(7):825-36. doi: 10.1016/s0092-8674(03)00985-1. Cell. 2003. PMID: 14697201
-
SUN-1 and ZYG-12, mediators of centrosome-nucleus attachment, are a functional SUN/KASH pair in Caenorhabditis elegans.Mol Biol Cell. 2009 Nov;20(21):4586-95. doi: 10.1091/mbc.e08-10-1034. Epub 2009 Sep 16. Mol Biol Cell. 2009. PMID: 19759181 Free PMC article.
-
LET-711, the Caenorhabditis elegans NOT1 ortholog, is required for spindle positioning and regulation of microtubule length in embryos.Mol Biol Cell. 2006 Nov;17(11):4911-24. doi: 10.1091/mbc.e06-02-0107. Epub 2006 Sep 13. Mol Biol Cell. 2006. PMID: 16971515 Free PMC article.
-
Centrosomes: hooked on the nucleus.Curr Biol. 2004 Apr 6;14(7):R268-70. doi: 10.1016/j.cub.2004.03.020. Curr Biol. 2004. PMID: 15062118 Review.
-
Regulation of microtubule nucleation mediated by γ-tubulin complexes.Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review.
Cited by
-
A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization.Curr Biol. 2012 Apr 10;22(7):575-82. doi: 10.1016/j.cub.2012.02.044. Epub 2012 Mar 15. Curr Biol. 2012. PMID: 22425160 Free PMC article.
-
Caenorhabditis elegans ortholog of the p24/p22 subunit, DNC-3, is essential for the formation of the dynactin complex by bridging DNC-1/p150(Glued) and DNC-2/dynamitin.Genes Cells. 2010 Nov;15(11):1145-57. doi: 10.1111/j.1365-2443.2010.01451.x. Epub 2010 Oct 21. Genes Cells. 2010. PMID: 20964796 Free PMC article.
-
LIN-41 inactivation leads to delayed centrosome elimination and abnormal chromosome behavior during female meiosis in Caenorhabditis elegans.Mol Biol Cell. 2016 Mar 1;27(5):799-811. doi: 10.1091/mbc.E15-10-0713. Epub 2016 Jan 13. Mol Biol Cell. 2016. PMID: 26764090 Free PMC article.
-
Microtubule organization, dynamics and functions in differentiated cells.Development. 2017 Sep 1;144(17):3012-3021. doi: 10.1242/dev.153171. Development. 2017. PMID: 28851722 Free PMC article. Review.
-
Control of oocyte meiotic maturation in C. elegans.Semin Cell Dev Biol. 2018 Dec;84:90-99. doi: 10.1016/j.semcdb.2017.12.005. Epub 2017 Dec 26. Semin Cell Dev Biol. 2018. PMID: 29242146 Free PMC article. Review.
References
-
- Bugnard E., Zaal K.J., Ralston E. 2005. Reorganization of microtubule nucleation during muscle differentiation.Cell Motil. Cytoskeleton. 60:1–13 - PubMed
-
- Dammermann A., Muller-Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. 2004. Centriole assembly requires both centriolar and pericentriolar material proteins.Dev. Cell. 7:815–829 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

