Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages
- PMID: 19635843
- PMCID: PMC2717652
- DOI: 10.1083/jcb.200903070
Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages
Abstract
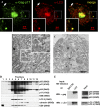
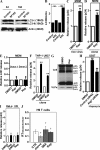
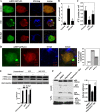
Autophagy is a cytoplasmic degradative pathway that can participate in biosynthetic processes, as in the yeast Cvt pathway, but is more commonly known for its functions in removing damaged or surplus organelles and macromolecular complexes. Here, we find that autophagy intersects with human immunodeficiency virus (HIV) biogenesis, mirroring the above dichotomy. Early, nondegradative stages of autophagy promoted HIV yields. HIV Gag-derived proteins colocalized and interacted with the autophagy factor LC3, and autophagy promoted productive Gag processing. Nevertheless, when autophagy progressed through maturation stages, HIV was degraded. This, however, does not occur, as the HIV protein Nef acts as an antiautophagic maturation factor through interactions with the autophagy regulatory factor Beclin 1, thus protecting HIV from degradation. The dual interaction of HIV with the autophagy pathway enhances viral yields by using the early stages while inhibiting the late stages of autophagy. The role of Nef in the latter process enhances yields of infectious HIV and may be of significance for progression to clinical AIDS.
Figures



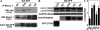
References
-
- Barre-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vezinet-Brun F., Rouzioux C., et al. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS).Science. 220:868–871 - PubMed
-
- Bentham M., Mazaleyrat S., Harris M. 2006. Role of myristoylation and N-terminal basic residues in membrane association of the human immunodeficiency virus type 1 Nef protein.J. Gen. Virol. 87:563–571 - PubMed
-
- Birmingham C.L., Smith A.C., Bakowski M.A., Yoshimori T., Brumell J.H. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole.J. Biol. Chem. 281:11374–11383 - PubMed
-
- Birmingham C.L., Canadien V., Kaniuk N.A., Steinberg B.E., Higgins D.E., Brumell J.H. 2008. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles.Nature. 451:350–354 - PubMed
-
- Brass A.L., Dykxhoorn D.M., Benita Y., Yan N., Engelman A., Xavier R.J., Lieberman J., Elledge S.J. 2008. Identification of host proteins required for HIV infection through a functional genomic screen.Science. 319:921–926 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

