The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones
- PMID: 19635855
- PMCID: PMC2717693
- DOI: 10.1085/jgp.200910232
The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones
Abstract
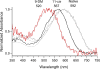
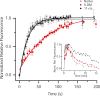
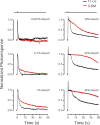
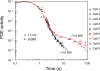
Cone photoreceptors of the vertebrate retina terminate their response to light much faster than rod photoreceptors. However, the molecular mechanisms underlying this rapid response termination in cones are poorly understood. The experiments presented here tested two related hypotheses: first, that the rapid decay rate of metarhodopsin (Meta) II in red-sensitive cones depends on interactions between the 9-methyl group of retinal and the opsin part of the pigment molecule, and second, that rapid Meta II decay is critical for rapid recovery from saturation of red-sensitive cones after exposure to bright light. Microspectrophotometric measurements of pigment photolysis, microfluorometric measurements of retinol production, and single-cell electrophysiological recordings of flash responses of salamander cones were performed to test these hypotheses. In all cases, cones were bleached and their visual pigment was regenerated with either 11-cis retinal or with 11-cis 9-demethyl retinal, an analogue of retinal lacking the 9-methyl group. Meta II decay was four to five times slower and subsequent retinol production was three to four times slower in red-sensitive cones lacking the 9-methyl group of retinal. This was accompanied by a significant slowing of the recovery from saturation in cones lacking the 9-methyl group after exposure to bright (>0.1% visual pigment photoactivated) but not dim light. A mathematical model of the turn-off process of phototransduction revealed that the slower recovery of photoresponse can be explained by slower Meta decay of 9-demethyl visual pigment. These results demonstrate that the 9-methyl group of retinal is required for steric chromophore-opsin interactions that favor both the rapid decay of Meta II and the rapid response recovery after exposure to bright light in red-sensitive cones.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

