Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells
- PMID: 19635862
- PMCID: PMC2722173
- DOI: 10.1084/jem.20082118
Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells
Abstract
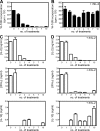
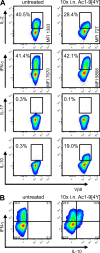
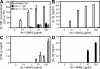
Regulation of the immune response to self- and foreign antigens is vitally important for limiting immune pathology associated with both infections and hypersensitivity conditions. Control of autoimmune conditions can be reinforced by tolerance induction with peptide epitopes, but the mechanism is not currently understood. Repetitive intranasal administration of soluble peptide induces peripheral tolerance in myelin basic protein (MBP)-specific TCR transgenic mice. This is characterized by the presence of anergic, interleukin (IL)-10-secreting CD4(+) T cells with regulatory function (IL-10 T reg cells). The differentiation pathway of peptide-induced IL-10 T reg cells was investigated. CD4(+) T cells became anergic after their second encounter with a high-affinity MBP peptide analogue. Loss of proliferative capacity correlated with a switch from the Th1-associated cytokines IL-2 and interferon (IFN)-gamma to the regulatory cytokine IL-10. Nevertheless, IL-10 T reg cells retained the capacity to produce IFN-gamma and concomitantly expressed T-bet, demonstrating their Th1 origin. IL-10 T reg cells suppressed dendritic cell maturation, prevented Th1 cell differentiation, and thereby created a negative feedback loop for Th1-driven immune pathology. These findings demonstrate that Th1 responses can be self-limiting in the context of peripheral tolerance to a self-antigen.
Figures





References
-
- Adler H.S., Kubsch S., Graulich E., Ludwig S., Knop J., Steinbrink K. 2007. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells.Blood. 109:4351–4359 - PubMed
-
- Anderson P.O., Manzo B.A., Sundstedt A., Minaee S., Symonds A., Khalid S., Rodriguez-Cabezas M.E., Nicolson K., Li S., Wraith D.C., et al. 2006. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance.Eur. J. Immunol. 36:1374–1385 - PMC - PubMed
-
- Anderson P.O., Sundstedt A., Yazici Z., Minaee S., O'Neill E.J., Woolf R., Nicolson K., Whitley N., Li L., Li S., et al. 2005. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells.J. Immunol. 174:310–319 - PubMed
-
- Andris F., Denanglaire S., de Mattia F., Urbain J., Leo O. 2004. Naive T cells are resistant to anergy induction by anti-CD3 antibodies.J. Immunol. 173:3201–3208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

