Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses
- PMID: 19635864
- PMCID: PMC2722183
- DOI: 10.1084/jem.20082460
Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses
Abstract
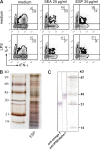
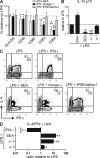
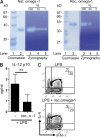
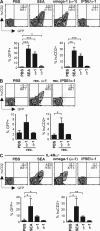
Soluble egg antigens of the parasitic helminth Schistosoma mansoni (S. mansoni egg antigen [SEA]) induce strong Th2 responses both in vitro and in vivo. However, the specific molecules that prime the development of Th2 responses have not been identified. We report that omega-1, a glycoprotein which is secreted from S. mansoni eggs and present in SEA, is capable of conditioning human monocyte-derived dendritic cells in vitro to drive T helper 2 (Th2) polarization with similar characteristics as whole SEA. Furthermore, using IL-4 dual reporter mice, we show that both natural and recombinant omega-1 alone are sufficient to generate Th2 responses in vivo, even in the absence of IL-4R signaling. Finally, omega-1-depleted SEA displays an impaired capacity for Th2 priming in vitro, but not in vivo, suggesting the existence of additional factors within SEA that can compensate for the omega-1-mediated effects. Collectively, we identify omega-1, a single component of SEA, as a potent inducer of Th2 responses.
Figures


Similar articles
-
The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1).J Exp Med. 2009 Aug 3;206(8):1681-90. doi: 10.1084/jem.20082462. Epub 2009 Jul 27. J Exp Med. 2009. PMID: 19635859 Free PMC article.
-
High-mannose glycans from Schistosoma mansoni eggs are important for priming of Th2 responses via Dectin-2 and prostaglandin E2.Front Immunol. 2024 Apr 29;15:1372927. doi: 10.3389/fimmu.2024.1372927. eCollection 2024. Front Immunol. 2024. PMID: 38742105 Free PMC article.
-
Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses.PLoS Biol. 2018 Apr 18;16(4):e2005504. doi: 10.1371/journal.pbio.2005504. eCollection 2018 Apr. PLoS Biol. 2018. PMID: 29668708 Free PMC article.
-
Schistosoma mansoni egg glycoproteins and C-type lectins of host immune cells: molecular partners that shape immune responses.Exp Parasitol. 2012 Sep;132(1):14-21. doi: 10.1016/j.exppara.2011.05.005. Epub 2011 May 15. Exp Parasitol. 2012. PMID: 21616068 Review.
-
Priming of the immune response by schistosome eggs.Parasite Immunol. 2005 Jul-Aug;27(7-8):265-70. doi: 10.1111/j.1365-3024.2005.00765.x. Parasite Immunol. 2005. PMID: 16138847 Review.
Cited by
-
Descriptive study on risk of increased morbidity of schistosomiasis and graft loss after liver transplantation.Rev Soc Bras Med Trop. 2024 Jul 29;57:e00201. doi: 10.1590/0037-8682-0097-2024. eCollection 2024. Rev Soc Bras Med Trop. 2024. PMID: 39082515 Free PMC article.
-
A comparative proteomics analysis of the egg secretions of three major schistosome species.Mol Biochem Parasitol. 2020 Nov;240:111322. doi: 10.1016/j.molbiopara.2020.111322. Epub 2020 Sep 19. Mol Biochem Parasitol. 2020. PMID: 32961206 Free PMC article.
-
A one-year unisexual Schistosoma mansoni infection causes pathologic organ alterations and persistent non-polarized T cell-mediated inflammation in mice.Front Immunol. 2022 Nov 24;13:1010932. doi: 10.3389/fimmu.2022.1010932. eCollection 2022. Front Immunol. 2022. PMID: 36505463 Free PMC article.
-
Acquired immune heterogeneity and its sources in human helminth infection.Parasitology. 2011 Feb;138(2):139-59. doi: 10.1017/S0031182010001216. Epub 2010 Oct 15. Parasitology. 2011. PMID: 20946693 Free PMC article. Review.
-
Schistosome Eggs Impair Protective Th1/Th17 Immune Responses Against Salmonella Infection.Front Immunol. 2018 Nov 14;9:2614. doi: 10.3389/fimmu.2018.02614. eCollection 2018. Front Immunol. 2018. PMID: 30487793 Free PMC article.
References
-
- Cervi L., MacDonald A.S., Kane C., Dzierszinski F., Pearce E.J. 2004. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses.J. Immunol. 172:2016–2020 - PubMed
-
- Cua D.J., Stohlman S.A. 1997. In vivo effects of T helper cell type 2 cytokines on macrophage antigen-presenting cell induction of T helper subsets.J. Immunol. 159:5834–5840 - PubMed
-
- de Jong E.C., Vieira P.L., Kalinski P., Schuitemaker J.H., Tanaka Y., Wierenga E.A., Yazdanbakhsh M., Kapsenberg M.L. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals.J. Immunol. 168:1704–1709 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

