SCID dogs: similar transplant potential but distinct intra-uterine growth defects and premature replicative senescence compared with SCID mice
- PMID: 19635917
- PMCID: PMC4047667
- DOI: 10.4049/jimmunol.0801406
SCID dogs: similar transplant potential but distinct intra-uterine growth defects and premature replicative senescence compared with SCID mice
Abstract
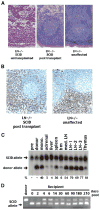
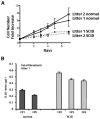
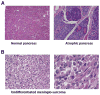
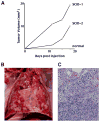
We have previously described DNA-dependent protein kinase (DNA-PKcs) mutations in horses and dogs that result in deficits in V(D)J recombination, DNA repair, and SCID. In this paper, we document substantial developmental growth defects in DNA-PKcs-deficient dogs that are not apparent in SCID mice. Fibroblast cell strains derived from either fetal or adult SCID dogs proliferate poorly in culture and undergo premature replicative senescence, somewhat reminiscent of cells derived from Ku-deficient mice. A limited number of animals have been immune reconstituted (by bone marrow transplantation) so that they can be maintained in a normal environment for long periods. Several of these animals have developed conditions associated with premature ageing at 2-3 years of age, roughly 20% of their expected lifespan. These conditions include intestinal malabsorption and primary neural cell neoplasia. These results suggest that DNA-PKcs deficiency is not tolerated equally in all species, perhaps providing insight into why DNA-PKcs deficiency has not been observed in humans. Finally, this study demonstrates the feasibility of maintaining SCID dogs for extended periods of time and documents their utility for bone marrow transplantation studies and as hosts for the propagation of xenografts. In sum, SCID dogs may present researchers with new possibilities for the development of animal models of human disease.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


References
-
- Fischer A, Le Deist F, Hacein-Bey-Abina S, Andre-Schmutz I, de Basile SG, de Villartay JP, Cavazzana-Calvo M. Severe combined immunodeficiency: a model disease for molecular immunology and therapy. Immunol Rev. 2005;203:98–109. - PubMed
-
- Shin EK, Perryman LE, Meek K. A kinase-negative mutation of DNA-PK(CS) in equine SCID results in defective coding and signal joint formation. J Immunol. 1997;158:3565–3569. - PubMed
-
- Taccioli GE, Rathbun G, Oltz E, Stamato T, Jeggo PA, Alt FW. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

