Termination of atrial fibrillation using pulsed low-energy far-field stimulation
- PMID: 19635972
- PMCID: PMC2867100
- DOI: 10.1161/CIRCULATIONAHA.108.825091
Termination of atrial fibrillation using pulsed low-energy far-field stimulation
Abstract
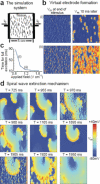
Background: Electrically based therapies for terminating atrial fibrillation (AF) currently fall into 2 categories: antitachycardia pacing and cardioversion. Antitachycardia pacing uses low-intensity pacing stimuli delivered via a single electrode and is effective for terminating slower tachycardias but is less effective for treating AF. In contrast, cardioversion uses a single high-voltage shock to terminate AF reliably, but the voltages required produce undesirable side effects, including tissue damage and pain. We propose a new method to terminate AF called far-field antifibrillation pacing, which delivers a short train of low-intensity electric pulses at the frequency of antitachycardia pacing but from field electrodes. Prior theoretical work has suggested that this approach can create a large number of activation sites ("virtual" electrodes) that emit propagating waves within the tissue without implanting physical electrodes and thereby may be more effective than point-source stimulation.
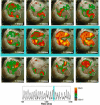
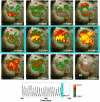
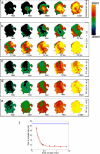
Methods and results: Using optical mapping in isolated perfused canine atrial preparations, we show that a series of pulses at low field strength (0.9 to 1.4 V/cm) is sufficient to entrain and subsequently extinguish AF with a success rate of 93% (69 of 74 trials in 8 preparations). We further demonstrate that the mechanism behind far-field antifibrillation pacing success is the generation of wave emission sites within the tissue by the applied electric field, which entrains the tissue as the field is pulsed.
Conclusions: AF in our model can be terminated by far-field antifibrillation pacing with only 13% of the energy required for cardioversion. Further studies are needed to determine whether this marked reduction in energy can increase the effectiveness and safety of terminating atrial tachyarrhythmias clinically.
Figures



References
-
- Waktare JE. Cardiology patient page. Atrial fibrillation. Circulation. 2002;106:14–16. - PubMed
-
- Heart Disease and Stroke Statistics—2007 Update. Circulation. 2007;115:e69–e171. - PubMed
-
- Nattel S, Opie LH. Controversies in atrial fibrillation. Lancet. 2006;367:262–272. - PubMed
-
- Wijffels M, Kirchhof C, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. - PubMed
-
- Ideker RE, Zhou X, Knisley SB. Correlation among fibrillation, defibrillation, and cardiac pacing. Pacing Clin Electrophysiol. 1995;18:512–525. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

