Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411
- PMID: 19636002
- PMCID: PMC2734421
- DOI: 10.1200/JCO.2009.21.8529
Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411
Abstract
Purpose: The primary objective of this study was to assess the 1-year survival of patients with locally advanced, unresectable pancreatic cancer treated with the combination of bevacizumab, capecitabine, and radiation. Secondary end points were toxicity, progression-free survival (PFS), and response rate (RR).
Patients and methods: Patients with locally advanced pancreatic cancer without duodenal invasion were treated with 50.4 Gy per 28 fractions to the gross tumor with concurrent capecitabine 825 mg/m(2) orally twice daily on days of radiation and bevacizumab 5 mg/kg on days 1, 15, and 29 followed by maintenance gemcitabine 1 g/m(2) weekly for 3 weeks and bevacizumab 5 mg/kg every 2 weeks, both in 4-week cycles until progression. Treatment plans were reviewed for quality assurance (QA).
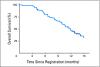
Results: Between January 2005 and February 2006, 82 eligible patients were treated. The median and 1-year survival rates were 11.9 months (95% CI, 9.9 to 14.0 months) and 47% (95% CI, 36% to 57%). Median PFS was 8.6 months (95% CI, 6.9 to 10.5), and RR was 26%. Overall, 35.4% of patients had grade 3 or greater treatment-related gastrointestinal toxicity (22.0% during chemoradiotherapy, 13.4% during maintenance chemotherapy). Unacceptable radiotherapy protocol deviations (ie, inappropriately generous volume contoured) correlated with grade 3 or greater gastrointestinal toxicity during chemoradiotherapy (45% v 18%; adjusted odds ratio, 3.7; 95% CI, 0.98 to 14.1; P = .05).
Conclusion: The addition of bevacizumab to chemoradiotherapy followed by bevacizumab and gemcitabine resulted in a similar median survival to previous Radiation Therapy Oncology Group studies in patients with locally advanced pancreatic cancer. Prospective QA may help limit toxicity in future trials.
Trial registration: ClinicalTrials.gov NCT00114179.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. - PubMed
-
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical