Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma
- PMID: 19636008
- PMCID: PMC4371138
- DOI: 10.1200/JCO.2008.21.3660
Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma
Abstract
Purpose: To assess safety and efficacy of presurgical bevacizumab in patients with metastatic renal cell carcinoma (mRCC), and to explore the hypothesis that pretreatment of patients with antiangiogenic therapy will select patients who benefit most from cytoreductive nephrectomy.
Patients and methods: Patients with newly diagnosed, clear cell mRCC whose primary tumors were considered resectable were enrolled. In this single-arm, phase II trial, patients received bevacizumab plus erlotinib (first patients, n = 23) or bevacizumab alone (n = 27 patients) for 8 weeks followed by restaging. If patients demonstrated progressive disease and had declining performance statuses after 8 weeks, nephrectomy procedures were deferred. Postoperatively, patients continued on the study drug or drugs if disease stabilization or regression had occurred.
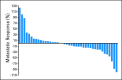
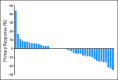
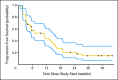
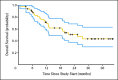
Results: Between March 2005 and March 2008, 52 patients were enrolled on study, and 50 were included in the analysis. By Memorial Sloan-Kettering Cancer Center criteria, 82% of patients had intermediate-risk, and 18% had poor-risk, features. Forty-two patients underwent nephrectomy. Median progression-free survival was 11.0 months (95% CI, 5.5 to 15.6 months). Median overall survival was 25.4 months (95% CI, 11.4 months to not estimable). Two perioperative deaths occurred; neither was attributable to study drug. Wound dehiscence resulted in treatment discontinuation for three patients and treatment delay for two others.
Conclusion: Presurgical treatment with bevacizumab therapy yields clinical outcomes comparable to post-surgical treatment with antiangiogenic therapy in patients with mRCC, but it may result in wound-healing delays. Prospective, randomized trials to test the use of presurgical therapy as a method to select appropriate patients for cytoreductive nephrectomy are warranted.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. - PubMed
-
- Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999;17:2859–2867. - PubMed
-
- McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. - PubMed
-
- Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical