Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma
- PMID: 19636009
- PMCID: PMC2734428
- DOI: 10.1200/JCO.2008.21.3496
Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma
Abstract
Purpose: To evaluate the safety and efficacy of high-dose [(131)I]metaiodobenzylguanidine ([(131)I]MIBG) in the treatment of malignant pheochromocytoma (PHEO) and paraganglioma (PGL).
Methods: Fifty patients with metastatic PHEO or PGL, age 10 to 64 years, were treated with [(131)I]MIBG doses ranging from 492 to 1,160 mCi (median, 12 mCi/kg). Cumulative [(131)I]MIBG administered ranged from 492 to 3,191 mCi. Autologous hematopoietic stem cells were collected and cryopreserved before treatment with [(131)I]MIBG greater than 12 mCi/kg or with a total dose greater than 500 mCi. Sixty-nine [(131)I]MIBG infusions were given, which included infusions to 35 patients treated once and infusions to 15 patients who received two or three treatments. Response was evaluated by [(123)I]MIBG scans, computed tomography/magnetic resonance imaging, urinary catecholamines/metanephrines, and chromogranin A.
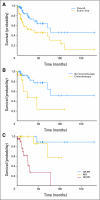
Results: The overall complete response (CR) plus partial response (PR) rate in 49 evaluable patients was 22%. Additionally, 35% of patients achieved a CR or PR in at least one measure of response without progressive disease, and 8% of patients maintained stable disease for greater than 12 months. Thirty-five percent of patients experienced progressive disease within 1 year after therapy. The estimated 5-year overall survival rate was 64%. Toxicities included grades 3 to 4 neutropenia (87%) and thrombocytopenia (83%). Grades 3 to 4 nonhematologic toxicity included acute respiratory distress syndrome (n = 2), bronchiolitis obliterans organizing pneumonia (n = 2), pulmonary embolism (n = 1), fever with neutropenia (n = 7), acute hypertension (n = 10), infection (n = 2), myelodysplastic syndrome (n = 2), and hypogonadism (n = 4).
Conclusion: Although serious toxicity may occur, the survival and response rates achieved with high-dose [(131)I]MIBG suggest its utility in the management of selected patients with metastatic PHEO and PGL.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG).Ann N Y Acad Sci. 2006 Aug;1073:465-90. doi: 10.1196/annals.1353.050. Ann N Y Acad Sci. 2006. PMID: 17102115 Clinical Trial.
-
High-dose 131I-metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma.Cancer. 2003 Jul 15;98(2):239-48. doi: 10.1002/cncr.11518. Cancer. 2003. PMID: 12872341
-
Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid.Cancer Biother Radiopharm. 2009 Aug;24(4):469-75. doi: 10.1089/cbr.2008.0584. Cancer Biother Radiopharm. 2009. PMID: 19694582 Clinical Trial.
-
High-Specific-Activity-131I-MIBG versus 177Lu-DOTATATE Targeted Radionuclide Therapy for Metastatic Pheochromocytoma and Paraganglioma.Clin Cancer Res. 2021 Jun 1;27(11):2989-2995. doi: 10.1158/1078-0432.CCR-20-3703. Epub 2021 Mar 8. Clin Cancer Res. 2021. PMID: 33685867 Free PMC article. Review.
-
The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients.J Endocrinol Invest. 1997 Dec;20(11):648-58. doi: 10.1007/BF03348026. J Endocrinol Invest. 1997. PMID: 9492103 Review.
Cited by
-
Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma.Curr Oncol Rep. 2013 Aug;15(4):356-71. doi: 10.1007/s11912-013-0320-x. Curr Oncol Rep. 2013. PMID: 23674235 Review.
-
Molecular and therapeutic advances in the diagnosis and management of malignant pheochromocytomas and paragangliomas.Oncologist. 2013;18(4):391-407. doi: 10.1634/theoncologist.2012-0410. Epub 2013 Apr 10. Oncologist. 2013. PMID: 23576482 Free PMC article. Review.
-
Patient with inoperable pheochromocytoma.Curr Oncol. 2015 Jun;22(3):e216-9. doi: 10.3747/co.22.2324. Curr Oncol. 2015. PMID: 26089731 Free PMC article.
-
Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: a pilot study.Eur J Nucl Med Mol Imaging. 2022 Apr;49(5):1595-1606. doi: 10.1007/s00259-021-05632-5. Epub 2021 Nov 27. Eur J Nucl Med Mol Imaging. 2022. PMID: 34837103 Free PMC article.
-
Intraoperative radiofrequency ablation for unresectable abdominal paraganglioma: a case report.Front Endocrinol (Lausanne). 2024 Apr 15;15:1346052. doi: 10.3389/fendo.2024.1346052. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38686210 Free PMC article.
References
-
- Fitzgerald PA, Goldsby RE, Huberty JP, et al. Malignant pheochromocytomas and paragangliomas: A phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. - PubMed
-
- Ahlman H. Malignant pheochromocytoma: State of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–464. - PubMed
-
- Safford SD, Coleman RE, Gockerman JP, et al. Iodine -131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery. 2003;134:956–962. discussion 962–963. - PubMed
-
- Rose B, Matthay KK, Price D, et al. High-dose 131I-metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma. Cancer. 2003;98:239–248. - PubMed
-
- Matthay KK, Panina C, Huberty J, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42:1713–1721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

