Accelerated approval of cancer drugs: improved access to therapeutic breakthroughs or early release of unsafe and ineffective drugs?
- PMID: 19636013
- PMCID: PMC2744277
- DOI: 10.1200/JCO.2008.21.1961
Accelerated approval of cancer drugs: improved access to therapeutic breakthroughs or early release of unsafe and ineffective drugs?
Abstract
Purpose: Accelerated approval (AA) was initiated by the US Food and Drug Administration (FDA) to shorten development times of drugs for serious medical illnesses. Sponsors must confirm efficacy in postapproval trials. Confronted with several drugs that received AA on the basis of phase II trials and for which confirmatory trials were incomplete, FDA officials have encouraged sponsors to design AA applications on the basis of interim analyses of phase III trials.
Methods: We reviewed data on orphan drug status, development time, safety, and status of confirmatory trials of AAs and regular FDA approvals of new molecular entities (NMEs) for oncology indications since 1995.
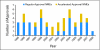
Results: Median development times for AA NMEs (n = 19 drugs) and regular-approval oncology NMEs (n = 32 drugs) were 7.3 and 7.2 years, respectively. Phase III trials supported efficacy for 75% of regular-approval versus 26% of AA NMEs and for 73% of non-orphan versus 45% of orphan drug approvals. AA accounted for 78% of approvals for oncology NMEs between 2001 and 2003 but accounted for 32% in more recent years. Among AA NMEs, confirmatory trials were nine-fold less likely to be completed for orphan drug versus non-orphan drug indications. Postapproval, black box warnings were added to labels for four oncology NMEs (17%) that had received AA and for two oncology NMEs (9%) that had received regular approval.
Conclusion: AA oncology NMEs are safe and effective, although development times are not accelerated. A return to endorsing phase II trial designs for AA for oncology NMEs, particularly for orphan drug indications, may facilitate timely FDA approval of novel cancer drugs.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Accelerated approval and oncology drug development timelines.J Clin Oncol. 2010 May 10;28(14):e226-7; author reply e228. doi: 10.1200/JCO.2009.26.2121. Epub 2010 Mar 1. J Clin Oncol. 2010. PMID: 20194846 No abstract available.
References
-
- Clinton W, Gore A. Reinventing the regulation of cancer drugs: National performance review. 1996 http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4191B1_01_03-Reinve....
-
- US Food and Drug Administration. Silver Spring, MD: US Food and Drug Administration; 2008. 21 Code of Federal Regulations, Part 314.510.
-
- Roberts TG, Jr, Chabner BA. Beyond fast track for drug approvals. N Engl J Med. 2004;351:501–505. - PubMed
-
- Oncologic Drugs Advisory Committee Meeting Transcript for March 12, 2003. Department of Health and Human Services; Food and Drug Administration Center for Drug Evaluation and Research. 2005
-
- Dagher R, Johnson J, Williams G, et al. Accelerated approval of oncology products: A decade of experience. J Natl Cancer Inst. 2004;96:1500–1509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

