Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer
- PMID: 19636017
- PMCID: PMC2734418
- DOI: 10.1200/JCO.2008.19.9968
Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer
Abstract
Purpose: Prostatic acid phosphatase (PAP) is a prostate tumor antigen. We have previously demonstrated that a DNA vaccine encoding PAP can elicit antigen-specific CD8+ T cells in rodents. We report here the results of a phase I/IIa trial conducted with a DNA vaccine encoding human PAP in patients with stage D0 prostate cancer.
Patients and methods: Twenty-two patients were treated in a dose-escalation trial with 100 microg, 500 microg, or 1,500 microg plasmid DNA, coadministered intradermally with 200 microg granulocyte-macrophage colony-stimulating factor as a vaccine adjuvant, six times at 14-day intervals. All patients were observed for 1 year after treatment.
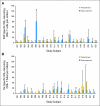
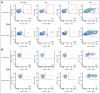
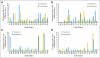
Results: No significant adverse events were observed. Three (14%) of 22 patients developed PAP-specific IFN gamma-secreting CD8+ T-cells immediately after the treatment course, as determined by enzyme-linked immunospot. Nine (41%) of 22 patients developed PAP-specific CD4+ and/or CD8+ T-cell proliferation. Antibody responses to PAP were not detected. Overall, the prostate-specific antigen (PSA) doubling time was observed to increase from a median 6.5 months pretreatment to 8.5 months on-treatment (P = .033), and 9.3 months in the 1-year post-treatment period (P = .054).
Conclusion: The demonstration that a DNA vaccine encoding PAP is safe, elicits an antigen-specific T-cell response, and may be associated with an increased PSA doubling time suggests that a multi-institutional phase II trial designed to evaluate clinical efficacy is warranted.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Immunotherapy for prostate cancer: walk, don't run.J Clin Oncol. 2009 Sep 1;27(25):4035-7. doi: 10.1200/JCO.2009.22.2299. Epub 2009 Jul 27. J Clin Oncol. 2009. PMID: 19635998 No abstract available.
-
Changes in PSA kinetics after DNA vaccine therapy-not so fast!J Clin Oncol. 2010 Feb 1;28(4):e58; author reply e59. doi: 10.1200/JCO.2009.26.3111. Epub 2009 Dec 14. J Clin Oncol. 2010. PMID: 20008614 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. - PubMed
-
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. - PubMed
-
- Slovin SF, Wilton AS, Heller G, et al. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. - PubMed
-
- Lee AK, Levy LB, Cheung R, et al. Prostate-specific antigen doubling time predicts clinical outcome and survival in prostate cancer patients treated with combined radiation and hormone therapy. Int J Radiat Oncol Biol Phys. 2005;63:456–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

