The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources
- PMID: 19636084
- PMCID: PMC3064075
- DOI: 10.1088/0031-9155/54/16/004
The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources
Abstract
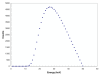
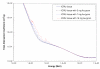
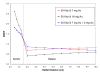
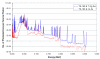
The preferential accumulation of gold nanoparticles within tumors and the increased photoelectric absorption due to the high atomic number of gold cooperatively account for the possibility of significant tumor dose enhancement during gold nanoparticle-aided radiation therapy (GNRT). Among the many conceivable ways to implement GNRT clinically, a brachytherapy approach using low-energy gamma-/x-ray sources (i.e. E(avg) < 100 keV) appears to be highly feasible and promising, because it may easily fulfill some of the technical and clinical requirements for GNRT. Therefore, the current study investigated the dosimetric feasibility of implementing GNRT using the following sources: (125)I, 50 kVp and (169)Yb. Specifically, Monte Carlo (MC) calculations were performed to determine the macroscopic dose enhancement factors (MDEF), defined as the ratio of the average dose in the tumor region with and without the presence of gold nanoparticles during the irradiation of the tumor, and the photo/Auger electron spectra within a tumor loaded with gold nanoparticles. The current study suggests that a significant tumor dose enhancement (e.g. >40%) could be achievable using (125)I, 50 kVp and (169)Yb sources and gold nanoparticles. When calculated at 1.0 cm from the center of the source within a tumor loaded with 18 mg Au g(-1), macroscopic dose enhancement was 116, 92 and 108% for (125)I, 50 kVp and (169)Yb, respectively. For a tumor loaded with 7 mg Au g(-1), it was 68, 57 and 44% at 1 cm from the center of the source for (125)I, 50 kVp and (169)Yb, respectively. The estimated MDEF values for (169)Yb were remarkably larger than those for (192)Ir, on average by up to about 70 and 30%, for 18 mg Au and 7 mg Au cases, respectively. The current MC study also shows a remarkable change in the photoelectron fluence and spectrum (e.g. more than two orders of magnitude) and a significant production (e.g. comparable to the number of photoelectrons) of the Auger electrons within the tumor region due to the presence of gold nanoparticles during low-energy gamma-/x-ray irradiation. The radiation sources considered in this study are currently available and tumor gold concentration levels considered in this investigation are deemed achievable. Therefore, the current results strongly suggest that GNRT can be successfully implemented via brachytherapy with low energy gamma-/x-ray sources, especially with a high dose rate (169)Yb source.
Figures





References
-
- Berger MJ, Hubbell JH, Seltzer SM, Chang J, Coursey JS, Sukumar R, Zucker DS. XCOM: Photon Cross Section Database. version 1.3. NIST; Gaithersburg, MD: 2005. http://physics.nist.gov/xcom.
-
- Birch MJ, Blowes RW. A contact x-ray therapy unit for intracavitary irradiation. Phys. Med. Biol. 1990;35:275–80. - PubMed
-
- Birch R, Marshall M, Ardran GM. Scientific Report. Vol. 30. Hospital Physicists Association; London: 1979. Catalogue of spectral data for diagnostic x-rays.
-
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54:631–51. - PubMed
-
- Butterworth KT, Wyer JA, Brennan-Fournet M, Latimer CJ, Shah MB, Currell FJ, Hirst DG. Variation of strand break yield for plasmid DNA irradiated with high-Z metal nanoparticles. Radiat. Res. 2008;170:381–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
