Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation
- PMID: 19636295
- PMCID: PMC3048782
- DOI: 10.1038/labinvest.2009.66
Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation
Abstract
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is characterized by chronic mucosal injury and the infiltration of inflammatory cells. Tumor suppressor FOXO3 regulates gene expression and its translocation to the cytosol leads to the abrogation of its transcriptional function. We have previously shown that bacterial infection regulates FOXO3 in intestinal epithelial cells and increases cytokine levels. As TNFalpha is a major contributor in intestinal inflammation, the aim of this study was to assess its effect on FOXO3 and FOXO3's contribution to intestinal inflammation in vitro and in vivo. TNFalpha induces the translocation of nuclear FOXO3 into the cytosol where it undergoes proteasomal degradation in human intestinal HT-29 cells. Proximally, the PI3K and IKK pathways mediate TNFalpha-induced FOXO3 phosphorylation. In FOXO3-silenced HT-29 cells, TNFalpha-induced IL-8 expression is increased approximately 83%. In vivo, Foxo3 is present in the nuclei and cytosol of colonic crypt epithelia. In DSS-induced colonic inflammation, Foxo3's nuclear localization is lost and it is only found in the cytosol. Consistent with a role for Foxo3 in colitis, Foxo3-deficient mice treated with DSS developed more severe colonic inflammation with an increased number of intraepithelial lymphocytes and PMNs infiltrated in the epithelia, than wild-type mice. In summary, TNFalpha inactivates FOXO3 in intestinal epithelia through the PI3K and IKK pathways and FOXO3 inactivation leads to the upregulation of IL-8 in vitro; in vivo Foxo3 is in the cytosol of inflamed colonic epithelia and Foxo3 deficiency leads to severe intestinal inflammation.
Conflict of interest statement
Figures



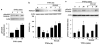
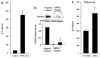


Similar articles
-
Intestinal inflammation requires FOXO3 and prostaglandin E2-dependent lipogenesis and elevated lipid droplets.Am J Physiol Gastrointest Liver Physiol. 2016 May 15;310(10):G844-54. doi: 10.1152/ajpgi.00407.2015. Epub 2016 Mar 11. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26968210 Free PMC article.
-
Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells.Infect Immun. 2008 Oct;76(10):4677-85. doi: 10.1128/IAI.00227-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678662 Free PMC article.
-
Tumor suppressor FOXO3 mediates signals from the EGF receptor to regulate proliferation of colonic cells.Am J Physiol Gastrointest Liver Physiol. 2011 Feb;300(2):G264-72. doi: 10.1152/ajpgi.00416.2010. Epub 2010 Nov 25. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21109589 Free PMC article.
-
IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G479-G489. doi: 10.1152/ajpgi.00208.2019. Epub 2019 Dec 2. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31790273 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
FOXO3a Gene Polymorphism Associated with Asthma in Indian Population.Mol Biol Int. 2015;2015:638515. doi: 10.1155/2015/638515. Epub 2015 Dec 9. Mol Biol Int. 2015. PMID: 26783460 Free PMC article.
-
Intestinal inflammation requires FOXO3 and prostaglandin E2-dependent lipogenesis and elevated lipid droplets.Am J Physiol Gastrointest Liver Physiol. 2016 May 15;310(10):G844-54. doi: 10.1152/ajpgi.00407.2015. Epub 2016 Mar 11. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26968210 Free PMC article.
-
The Association Between Forkhead Box Class O3A Gene Polymorphism and Psoriasis and Its Relationship with Psoriasis Severity.J Clin Aesthet Dermatol. 2022 Aug;15(8):22-26. J Clin Aesthet Dermatol. 2022. PMID: 36061485 Free PMC article.
-
High-fat diet induced leptin and Wnt expression: RNA-sequencing and pathway analysis of mouse colonic tissue and tumors.Carcinogenesis. 2017 Mar 1;38(3):302-311. doi: 10.1093/carcin/bgx001. Carcinogenesis. 2017. PMID: 28426873 Free PMC article.
-
The microRNA expression in crypt-top and crypt-bottom colonic epithelial cell populations demonstrates cell-type specificity and correlates with endoscopic activity in ulcerative colitis.J Crohns Colitis. 2024 Jul 18;18(12):2033-44. doi: 10.1093/ecco-jcc/jjae108. Online ahead of print. J Crohns Colitis. 2024. PMID: 39022905 Free PMC article.
References
-
- Podolsky DK. Inflammatory bowel disease. New Eng J Med. 2002;347:417–429. - PubMed
-
- Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastro. 1994;106:1455–1466. - PubMed
-
- Maeda M, Watanabe N, Neda H, et al. Serum tumor necrosis factor activity in inflammatory bowel disease. Immunopharm Immunotox. 1992;14:451–461. - PubMed
-
- Komatsu M, Kobayashi D, Saito K, et al. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297–1301. - PubMed
-
- van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastro. 1995;109:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

