CD47 regulates collagen I-induced cyclooxygenase-2 expression and intestinal epithelial cell migration
- PMID: 19636412
- PMCID: PMC2712095
- DOI: 10.1371/journal.pone.0006371
CD47 regulates collagen I-induced cyclooxygenase-2 expression and intestinal epithelial cell migration
Abstract
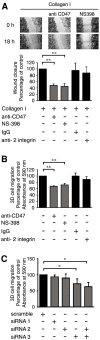
Increased epithelial cell expression of the cyclooxygenase-2 (COX-2) enzyme is a characteristic event of both inflammatory bowel disease and colon cancer. We here report the novel findings that collagen I-induced de novo synthesis of COX-2 in intestinal epithelial cells is inhibited by pertussis toxin (PTX) and by an inhibitory peptide selective for the heterotrimeric G alpha(i3)-protein. These findings could be explained by a regulatory involvement of the G-protein-dependent integrin-associated protein CD47. In support of this notion, we observed a collagen I-induced association between CD47 and alpha2 integrins. This association was reduced by a blocking anti-CD47 antibody but not by PTX or a control anti-beta2 antibody. Furthermore, a blocking antibody against CD47, dominant negative CD47 or specific siRNA knock down of CD47, significantly reduced collagen I-induced COX-2 expression. COX-2 has previously been shown to regulate intestinal epithelial cell adhesion and migration. Morphological analysis of intestinal cells adhering to collagen I revealed a co-localisation of CD47 and alpha2 integrins to non-apoptotic membrane blebs enriched in Rho A and F-actin. The blocking CD47 antibody, PTX and a selective COX-2 inhibitor, dramatically inhibited the formation of these blebs. In accordance, migration of these cells on a collagen I-coated surface or through a collagen I gel were significantly reduced by the CD47 blocking antibody, siRNA knock down of CD47 and the COX-2 inhibitor NS-398. In conclusion, we present novel data that identifies the G-protein-dependent CD47 protein as a key regulator of collagen I-induced COX-2 expression and a promoter of intestinal epithelial cell migration.
Conflict of interest statement
Figures




Similar articles
-
Alpha2beta1 integrin signalling enhances cyclooxygenase-2 expression in intestinal epithelial cells.J Cell Physiol. 2006 Dec;209(3):950-8. doi: 10.1002/jcp.20796. J Cell Physiol. 2006. PMID: 16972245
-
CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia.J Cell Biol. 1996 Feb;132(3):437-50. doi: 10.1083/jcb.132.3.437. J Cell Biol. 1996. PMID: 8636220 Free PMC article.
-
Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha.J Immunol. 2004 Feb 15;172(4):2578-85. doi: 10.4049/jimmunol.172.4.2578. J Immunol. 2004. PMID: 14764731
-
The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47.J Biol Chem. 2001 Oct 26;276(43):40156-66. doi: 10.1074/jbc.M104138200. Epub 2001 Jul 30. J Biol Chem. 2001. PMID: 11479293
-
Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration.J Biol Chem. 2002 Mar 22;277(12):10028-36. doi: 10.1074/jbc.M109720200. Epub 2002 Jan 15. J Biol Chem. 2002. PMID: 11792697
Cited by
-
Cell autonomous functions of CD47 in regulating cellular plasticity and metabolic plasticity.Cell Death Differ. 2024 Oct;31(10):1255-1266. doi: 10.1038/s41418-024-01347-w. Epub 2024 Jul 23. Cell Death Differ. 2024. PMID: 39039207 Free PMC article. Review.
-
Silencing of microRNA families by seed-targeting tiny LNAs.Nat Genet. 2011 Mar 20;43(4):371-8. doi: 10.1038/ng.786. Nat Genet. 2011. PMID: 21423181 Free PMC article.
-
Checkpoint CD47 Function On Tumor Metastasis And Immune Therapy.Onco Targets Ther. 2019 Nov 4;12:9105-9114. doi: 10.2147/OTT.S220196. eCollection 2019. Onco Targets Ther. 2019. PMID: 31806995 Free PMC article. Review.
-
CD47 Promotes Human Glioblastoma Invasion Through Activation of the PI3K/Akt Pathway.Oncol Res. 2019 Mar 29;27(4):415-422. doi: 10.3727/096504018X15155538502359. Epub 2018 Jan 10. Oncol Res. 2019. PMID: 29321087 Free PMC article.
-
CD47 (Cluster of Differentiation 47).Atlas Genet Cytogenet Oncol Haematol. 2021;25(2):83-102. Atlas Genet Cytogenet Oncol Haematol. 2021. PMID: 34707698 Free PMC article.
References
-
- Ristimaki A. Cyclooxygenase 2: from inflammation to carcinogenesis. Novartis Found Symp. 2004;256 - PubMed
-
- DuBois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. - PubMed
-
- Williams CS, Shattuck-Brandt RL, DuBois RN. The role of COX-2 in intestinal cancer. Expert Opin Investig Drugs. 1999;8:1–12. - PubMed
-
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. - PubMed
-
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol. 2004;287:G7–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

