Immune reactions against elongation factor 2 kinase: specific pathogenesis of gastric ulcer from Helicobacter pylori infection
- PMID: 19636416
- PMCID: PMC2712636
- DOI: 10.1155/2009/850623
Immune reactions against elongation factor 2 kinase: specific pathogenesis of gastric ulcer from Helicobacter pylori infection
Abstract
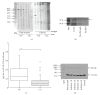
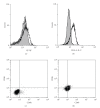
Helicobacter pylori (H. pylori) infection is a definite causative factor for gastric ulcers (GUs). In the present study we detected a specific antigen of gastric epithelial cells (HGC-27) using cell ELISA, which was recognized by the sera of GU patients (n = 20) but not in patients with chronic gastritis (CG; n = 20) or in healthy volunteers (HC; n = 10). This antigen was over-expressed by a stressful (heat-stressed) environment, and was identified as elongation factor 2 kinase (EF-2K) by western blotting. The GU patients' lymphocytes stimulated by H. pylori specifically disrupted heat-stressed HGC-27 cells in a cytotoxic assay. In flow cytometry, the effector cells (lymphocytes) from GU patients were significantly differentiated to T helper type 1 lymphocyte (Th1) and cytotoxic T lymphocyte (CTL) as opposed to those from CG patients. The target cells (HGC-27) expressed EF-2K and MHC-class I together with costimulatory molecules from heat stress. This antigen specific immune mechanism could have a prominent role in the pathogenesis of GU.
Figures



References
-
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric campylobacter. Medical Journal of Australia. 1985;142(8):436–439. - PubMed
-
- Marshall BJ, McGechie DB, Rogers PA, Glancy RJ. Pyloric campylobacter infection and gastroduodenal disease. The Medical Journal of Australia. 1985;142(8):439–444. - PubMed
-
- Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Annals of Internal Medicine. 1992;116(9):705–708. - PubMed
-
- Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England Journal of Medicine. 1991;325(16):1127–1131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

