SlipChip
- PMID: 19636458
- PMCID: PMC2719824
- DOI: 10.1039/b908978k
SlipChip
Abstract
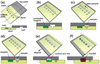
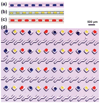
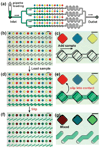
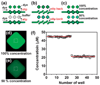
The SlipChip is a microfluidic device designed to perform multiplexed microfluidic reactions without pumps or valves. The device has two plates in close contact. The bottom plate contains wells preloaded with many reagents; in this paper plates with 48 reagents were used. These wells are covered by the top plate that acts as a lid for the wells with reagents. The device also has a fluidic path, composed of ducts in the bottom plate and wells in the top plate, which is connected only when the top and bottom plate are aligned in a specific configuration. Sample can be added into the fluidic path, filling both wells and ducts. Then, the top plate is "slipped", or moved, relative to the bottom plate so the complementary patterns of wells in both plates overlap, exposing the sample-containing wells of the top plate to the reagent-containing wells of the bottom plate, and enabling diffusion and reactions. Between the two plates, a lubricating layer of fluorocarbon was used to facilitate relative motion of the plates. This paper implements this approach on a nanoliter scale using devices fabricated in glass. Stability of preloaded solutions, control of loading, and lack of cross-contamination were tested using fluorescent dyes. Functionality of the device was illustrated via crystallization of a model membrane protein. Fabrication of this device is simple and does not require a bonding step. This device requires no pumps or valves and is applicable to resource-poor settings. Overall, this device should be valuable for multiplexed applications that require exposing one sample to many reagents in small volumes. One may think of the SlipChip as an easy-to-use analogue of a preloaded multi-well plate, or a preloaded liquid-phase microarray.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

