Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group
- PMID: 19637348
- PMCID: PMC2783515
- DOI: 10.1002/cncr.24566
Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group
Abstract
Background: The addition of liposomal muramyl tripeptide phosphatidylethanolamine (MTP-PE) to chemotherapy has been shown to improve overall survival in patients with nonmetastatic osteosarcoma (OS). The authors report the results of addition of liposomal MTP-PE to chemotherapy for patients with metastatic OS.
Methods: Intergroup-0133 was a prospective randomized phase 3 trial for the treatment of newly diagnosed patients with OS. The authors compared 3-drug chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate (Regimen A) to the same 3 drugs with the addition of ifosfamide (Regimen B). The addition of liposomal MTP-PE to chemotherapy was evaluated.
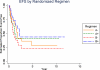
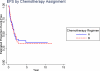
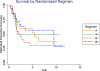
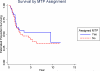
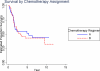
Results: Five-year event-free survival (EFS) for patients who received liposomal MTP-PE (n = 46) was 42% versus 26% for those who did not (n = 45) (relative risk for liposomal MTP-PE, 0.72; P = .23; 95% confidence interval [CI], 0.42-1.2). The 5-year overall survival for patients who received MTP-PE versus no MTP-PE was 53% and 40%, respectively (relative risk for liposomal MTP-PE, 0.72; P = 0.27; 95% CI, 0.40-1.3). The comparison of Regimen A with Regimen B did not suggest a difference for EFS (35% vs 34%, respectively; relative risk for Regimen B, 1.07; P = .79; 95% CI, 0.62-1.8) or overall survival (52% vs 43%, respectively; relative risk for Regimen B, 1.1, P = .75; 95% CI, 0.61-2.0).
Conclusions: When the metastatic cohort was considered in isolation, the addition of liposomal MTP-PE to chemotherapy did not achieve a statistically significant improvement in outcome. However, the pattern of outcome is similar to the pattern in nonmetastatic patients.
Figures


References
-
- Seibel NL, Krailo M, Chen Z, Healey J, Breitfeld PP, Drachtman R, et al. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: children's Cancer Group (CCG) 7943. Cancer. 2007;109(8):1646–53. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–61. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Daw NC, Billups CA, Rodriguez-Galindo C, McCarville MB, Rao BN, Cain AM, et al. Metastatic osteosarcoma. Cancer. 2006;106(2):403–12. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- McTiernan A, Meyer T, Michelagnoli MP, Lewis I, Whelan JS. A phase I/II study of doxorubicin, ifosfamide, etoposide and interval methotrexate in patients with poor prognosis osteosarcoma. Pediatr Blood Cancer. 2006;46(3):345–50. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104(5):1100–9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

