Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate
- PMID: 19637353
- PMCID: PMC2782911
- DOI: 10.1002/cncr.24532
Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate
Abstract
Background: 3-bromopyruvate is an alkylating agent with antitumor activity. It is currently believed that blockade of adenosine triphosphate production from glycolysis and mitochondria is the primary mechanism responsible for this antitumor effect. The current studies uncovered a new and novel mechanism for the antitumor activity of 3-bromopyruvate.
Methods: The transport of 3-bromopyruvate by sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8), a tumor suppressor and a sodium (Na+)-coupled, electrogenic transporter for short-chain monocarboxylates, was studied using a mammalian cell expression and the Xenopus laevis oocyte expression systems. The effect of 3-bromopyruvate on histone deacetylases (HDACs) was monitored using the lysate of the human breast cancer cell line MCF7 and human recombinant HDAC isoforms as the enzyme sources. Cell viability was monitored by fluorescence-activated cell-sorting analysis and colony-formation assay. The acetylation status of histone H4 was evaluated by Western blot analysis.
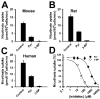
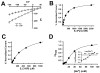
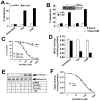
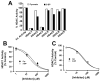
Results: 3-Bromopyruvate is a transportable substrate for SLC5A8, and that transport process is Na+-coupled and electrogenic. MCF7 cells did not express SLC5A8 and were not affected by 3-bromopyruvate. However, when transfected with SLC5A8 or treated with inhibitors of DNA methylation, these cells underwent apoptosis in the presence of 3-bromopyruvate. This cell death was associated with the inhibition of HDAC1/HDAC3. Studies with different isoforms of human recombinant HDACs identified HDAC1 and HDAC3 as the targets for 3-bromopyruvate.
Conclusions: 3-Bromopyruvate was transported into cells actively through the tumor suppressor SLC5A8, and the process was energized by an electrochemical Na+ gradient. Ectopic expression of the transporter in MCF7 cells led to apoptosis, and the mechanism involved the inhibition of HDAC1/HDAC3.
Copyright (c) 2009 American Cancer Society.
Figures

References
-
- Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. - PubMed
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. - PubMed
-
- Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nutr Metab Care. 2006;9:339–345. - PubMed
-
- Kim J, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. - PubMed
-
- Pedersen PL. The cancer cell's “power plants” as promising therapeutic targets: An overview. J Bioenerg Biomembr. 2007;39:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

