Age-prioritized use of antivirals during an influenza pandemic
- PMID: 19638194
- PMCID: PMC2728723
- DOI: 10.1186/1471-2334-9-117
Age-prioritized use of antivirals during an influenza pandemic
Abstract
Background: The WHO suggested that governments stockpile, as part of preparations for the next influenza pandemic, sufficient influenza antiviral drugs to treat approximately 25% of their populations. Our aim is two-fold: first, since in many countries the antiviral stockpile is well below this level, we search for suboptimal strategies based on treatment provided only to an age-dependent fraction of cases. Second, since in some countries the stockpile exceeds the suggested minimum level, we search for optimal strategies for post-exposure prophylactic treatment of close contacts of cases.
Methods: We used a stochastic, spatially structured individual-based model, considering explicit transmission in households, schools and workplaces, to simulate the spatiotemporal spread of an influenza pandemic in Italy and to evaluate the efficacy of interventions based on age-prioritized use of antivirals.
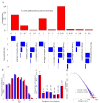
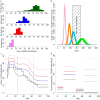
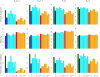
Results: Our results show that the antiviral stockpile required for treatment of cases ranges from 10% to 35% of the population for R0 in 1.4 - 3. No suboptimal strategies, based on treatment provided to an age-dependent fraction of cases, were found able to remarkably reduce both clinical attack rate and antiviral drugs needs, though they can contribute to largely reduce the excess mortality. Treatment of all cases coupled with prophylaxis provided to younger individuals is the only intervention resulting in a significant reduction of the clinical attack rate and requiring a relatively small stockpile of antivirals.
Conclusion: Our results strongly suggest that governments stockpile sufficient influenza antiviral drugs to treat approximately 25% of their populations, under the assumption that R0 is not much larger than 2. In countries where the number of antiviral stockpiled exceeds the suggested minimum level, providing prophylaxis to younger individuals is an option that could be taken into account in preparedness plans. In countries where the number of antivirals stockpiled is well below 25% of the population, priority should be decided based on age-specific case fatality rates. However, late detection of cases (administration of antivirals 48 hours after the clinical onset of symptoms) dramatically affects the efficacy of both treatment and prophylaxis.
Figures
Similar articles
-
Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy.PLoS Med. 2009 May 19;6(5):e1000085. doi: 10.1371/journal.pmed.1000085. Epub 2009 May 19. PLoS Med. 2009. PMID: 19440354 Free PMC article.
-
Choice of Antiviral Allocation Scheme for Pandemic Influenza Depends on Strain Transmissibility, Delivery Delay and Stockpile Size.Bull Math Biol. 2016 Feb;78(2):293-321. doi: 10.1007/s11538-016-0144-6. Epub 2016 Feb 3. Bull Math Biol. 2016. PMID: 26846916
-
Estimation of optimal antiviral stockpile for a novel influenza pandemic.J Infect Public Health. 2022 Jul;15(7):720-725. doi: 10.1016/j.jiph.2022.05.012. Epub 2022 May 26. J Infect Public Health. 2022. PMID: 35667304
-
Antivirals in the management of an influenza pandemic.Med J Aust. 2006 Nov 20;185(S10):S58-61. doi: 10.5694/j.1326-5377.2006.tb00709.x. Med J Aust. 2006. PMID: 17115954 Review.
-
The risk of seasonal and pandemic influenza: prospects for control.Clin Infect Dis. 2009 Jan 1;48 Suppl 1:S20-5. doi: 10.1086/591853. Clin Infect Dis. 2009. PMID: 19067611 Review.
Cited by
-
Connecting within and between-hosts dynamics in the influenza infection-staged epidemiological models with behavior change.J Coupled Syst Multiscale Dyn. 2015 Sep;3(3):233-243. doi: 10.1166/jcsmd.2015.1082. J Coupled Syst Multiscale Dyn. 2015. PMID: 29075652 Free PMC article.
-
Modeling rapidly disseminating infectious disease during mass gatherings.BMC Med. 2012 Dec 7;10:159. doi: 10.1186/1741-7015-10-159. BMC Med. 2012. PMID: 23217051 Free PMC article. Review.
-
Assessing the Role of Voluntary Self-Isolation in the Control of Pandemic Influenza Using a Household Epidemic Model.Int J Environ Res Public Health. 2015 Aug 18;12(8):9750-67. doi: 10.3390/ijerph120809750. Int J Environ Res Public Health. 2015. PMID: 26295248 Free PMC article.
-
Transmission potential and design of adequate control measures for Marburg hemorrhagic fever.PLoS One. 2012;7(12):e50948. doi: 10.1371/journal.pone.0050948. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251407 Free PMC article.
-
Comparing large-scale computational approaches to epidemic modeling: agent-based versus structured metapopulation models.BMC Infect Dis. 2010 Jun 29;10:190. doi: 10.1186/1471-2334-10-190. BMC Infect Dis. 2010. PMID: 20587041 Free PMC article.
References
-
- WHO. Influenza A(H1N1) – update 50. 2009. http://www.who.int/csr/don/2009_06_17/en/index.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

