Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis
- PMID: 19638304
- PMCID: PMC2760658
- DOI: 10.1016/j.neuroscience.2009.07.044
Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis
Abstract
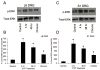
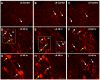
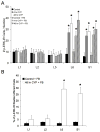
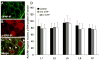
Extracellular signal-regulated kinases (ERK1 and ERK2) are phosphorylated in the nervous system after somatic or visceral stimulation or inflammation and play roles in central sensitization and pain hypersensitivity. ERK1/2 activation with cyclophosphamide (CYP)-induced cystitis has been demonstrated in urinary bladder and inhibitors of ERK1/2 phosphorylation reduce CYP-induced bladder hyperreflexia. In this study, we determined pERK1/2 expression and regulation in lumbosacral dorsal root ganglia (DRG) and spinal cord with CYP-induced cystitis (4 h, 48 h, chronic) using Western blotting and immunohistochemical techniques. Phosphorylated extracellular signal-regulated kinases (pERK1/2) expression was significantly (P< or =0.01) upregulated in L6 and S1 DRG with CYP-induced cystitis with the greatest upregulation occurring at 4 h. No changes in pERK1/2 expression were observed in L1, L2 or L5 DRG or in any spinal cord segment examined (L1, L2, L5-S1) with CYP-induced cystitis. Cytoplasmic pERK1/2-immunoreactivity (IR) and pericellular pERK1/2-IR was observed in all DRG examined from control rats and cytoplasmic pERK1/2-IR was significantly (P< or =0.01) increased in L6 and S1 DRG with 4 and 48 h CYP-induced cystitis. In contrast, pericellular pERK1/2-IR in DRG was not regulated by CYP-induced cystitis. A small percentage of bladder afferent cells in lumbosacral DRG expressed pERK1/2-IR in control rats; however, CYP-induced cystitis (48 h) significantly (P< or =0.01) increased the percentage of bladder afferent cells in the L6 and S1 DRG exhibiting pERK1/2-IR. These studies suggest that activation of the ERK pathway in lumbosacral DRG may play a role in neuroplasticity in micturition reflexes with CYP-induced cystitis.
Figures
Similar articles
-
Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways.J Comp Neurol. 2002 Dec 9;454(2):200-11. doi: 10.1002/cne.10447. J Comp Neurol. 2002. PMID: 12412144
-
Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis.J Comp Neurol. 2004 Feb 2;469(2):262-74. doi: 10.1002/cne.11009. J Comp Neurol. 2004. PMID: 14694538
-
Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis.Brain Res. 1999 Oct 9;844(1-2):174-87. doi: 10.1016/s0006-8993(99)01936-8. Brain Res. 1999. PMID: 10536274
-
Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis.J Chem Neuroanat. 2001 Mar;21(2):125-38. doi: 10.1016/s0891-0618(00)00115-0. J Chem Neuroanat. 2001. PMID: 11312054
-
Cross-talk and sensitization of bladder afferent nerves.Neurourol Urodyn. 2010;29(1):77-81. doi: 10.1002/nau.20817. Neurourol Urodyn. 2010. PMID: 20025032 Free PMC article. Review.
Cited by
-
Role of c-Jun N-terminal kinase (JNK) activation in micturition reflexes in cyclophosphamide (CYP)-induced cystitis in female rats.J Mol Neurosci. 2014 Nov;54(3):360-9. doi: 10.1007/s12031-014-0308-5. Epub 2014 Apr 26. J Mol Neurosci. 2014. PMID: 24763745 Free PMC article.
-
Hyperbaric oxygen therapy in a rat model of cyclophosphamide-induced hemorrhagic cystitis.Int Urol Nephrol. 2025 Jul 17. doi: 10.1007/s11255-025-04650-8. Online ahead of print. Int Urol Nephrol. 2025. PMID: 40670874
-
Effects of pharmacological neurotrophin receptor inhibition on bladder function in female mice with cyclophosphamide-induced cystitis.Front Urol. 2022;2:1037511. doi: 10.3389/fruro.2022.1037511. Epub 2022 Nov 8. Front Urol. 2022. PMID: 37701182 Free PMC article.
-
Adenosine A2A Receptor Agonist Polydeoxyribonucleotide Alleviates Interstitial Cystitis-Induced Voiding Dysfunction by Suppressing Inflammation and Apoptosis in Rats.J Inflamm Res. 2021 Feb 15;14:367-378. doi: 10.2147/JIR.S287346. eCollection 2021. J Inflamm Res. 2021. PMID: 33623409 Free PMC article.
-
Role of CXCR2 and TRPV1 in functional, inflammatory and behavioural changes in the rat model of cyclophosphamide-induced haemorrhagic cystitis.Br J Pharmacol. 2014 Jan;171(2):452-67. doi: 10.1111/bph.12467. Br J Pharmacol. 2014. PMID: 24117268 Free PMC article.
References
-
- Ahn M, Moon C, Lee Y, Koh CS, Kohyama K, Tanum N, Matsumoto Y, Kim HM, Kim SR, Shin T. Activation of extracellular signal-regulated kinases in the sciatic nerves of rats with experimental autoimmune neuritis. Neurosci Lett. 2004;372(1–2):57–61. - PubMed
-
- Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R125–134. - PubMed
-
- Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci. 2005;21(3):773–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

