Identification and functional characterization of cytoplasmic determinants of plasmid DNA nuclear import
- PMID: 19638341
- PMCID: PMC2785383
- DOI: 10.1074/jbc.M109.034850
Identification and functional characterization of cytoplasmic determinants of plasmid DNA nuclear import
Abstract
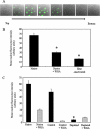
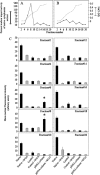
Import of exogenous plasmid DNA (pDNA) into mammalian cell nuclei represents a key intracellular obstacle to efficient non-viral gene delivery. This includes access of the pDNA to the nuclei of non-dividing cells where the presence of an intact nuclear membrane is limiting for gene transfer. Here we identify, isolate, and characterize, cytoplasmic determinants of pDNA nuclear import into digitonin-permeabilized HeLa cells. Depletion of putative DNA-binding proteins, on the basis of their ability to bind immobilized pDNA, abolished pDNA nuclear import supporting the critical role of cytoplasmic factors in this process. Elution of pDNA-bound proteins, followed by two-dimensional sodium dodecyl polyacrylamide gel electrophoresis identified several candidate DNA shuttle proteins. We show that two of these, NM23-H2, a ubiquitous c-Myc transcription-activating nucleoside diphosphate kinase, and the core histone H2B can both reconstitute pDNA nuclear import. Further, we demonstrate a significant increase in gene transfer in non-dividing HeLa cells transiently transfected with pDNA containing binding sequences from two of the DNA shuttle proteins, NM23-H2 and the homeobox transcription factor Chx10. These data support the hypothesis that exogenous pDNA binds to cytoplasmic shuttle proteins and is then translocated to the nucleus using the minimal import machinery. Importantly, increasing the binding of pDNA to shuttle proteins by re-engineering reporter plasmids with shuttle binding sequences enhances gene transfer. Increasing the potential for exogenously added pDNA to bind intracellular transport cofactors may enhance the potency of non-viral gene transfer.
Figures




Similar articles
-
Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences.J Biol Chem. 1999 Jul 30;274(31):22025-32. doi: 10.1074/jbc.274.31.22025. J Biol Chem. 1999. PMID: 10419528 Free PMC article.
-
Ultrasound microbubbles combined with the NFκB binding motif increase transfection efficiency by enhancing the cytoplasmic and nuclear import of plasmid DNA.Mol Med Rep. 2013 Nov;8(5):1439-45. doi: 10.3892/mmr.2013.1672. Epub 2013 Sep 9. Mol Med Rep. 2013. PMID: 24026477
-
Exploring the mechanism of plasmid DNA nuclear internalization with polymer-based vehicles.Mol Pharm. 2012 Aug 6;9(8):2256-67. doi: 10.1021/mp300142d. Epub 2012 Jul 9. Mol Pharm. 2012. PMID: 22715912 Free PMC article.
-
Emerging significance of plasmid DNA nuclear import in gene therapy.Adv Drug Deliv Rev. 2003 Jun 16;55(6):749-60. doi: 10.1016/s0169-409x(03)00050-4. Adv Drug Deliv Rev. 2003. PMID: 12788538 Review.
-
Cytoplasmic transport and nuclear import of plasmid DNA.Biosci Rep. 2017 Nov 29;37(6):BSR20160616. doi: 10.1042/BSR20160616. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 29054961 Free PMC article. Review.
Cited by
-
Electroporation-mediated gene delivery.Adv Genet. 2015;89:49-88. doi: 10.1016/bs.adgen.2014.10.003. Epub 2014 Dec 11. Adv Genet. 2015. PMID: 25620008 Free PMC article.
-
Ultrasound‑targeted microbubbles combined with a peptide nucleic acid binding nuclear localization signal mediate transfection of exogenous genes by improving cytoplasmic and nuclear import.Mol Med Rep. 2017 Dec;16(6):8819-8825. doi: 10.3892/mmr.2017.7681. Epub 2017 Oct 2. Mol Med Rep. 2017. PMID: 28990051 Free PMC article.
-
On the possible involvement of bovine serum albumin precursor in lipofection pathway.J Biosci. 2014 Mar;39(1):43-52. doi: 10.1007/s12038-014-9415-2. J Biosci. 2014. PMID: 24499789
-
Transcription factor plasmid binding modulates microtubule interactions and intracellular trafficking during gene transfer.Gene Ther. 2012 Mar;19(3):338-46. doi: 10.1038/gt.2011.96. Epub 2011 Jun 30. Gene Ther. 2012. PMID: 21716302 Free PMC article.
-
Membrane and nuclear permeabilization by polymeric pDNA vehicles: efficient method for gene delivery or mechanism of cytotoxicity?Mol Pharm. 2012 Mar 5;9(3):523-38. doi: 10.1021/mp200368p. Epub 2012 Feb 1. Mol Pharm. 2012. PMID: 22175236 Free PMC article.
References
-
- Nishikawa M., Huang L. (2001) Hum. Gene Ther. 12, 861–870 - PubMed
-
- Görlich. D., Kutay U. (1999) Annu. Rev. Cell. Dev. Biol. 15, 607–660 - PubMed
-
- Tseng W. C., Haselton F. R., Giorgio T. D. (1999) Biochim. Biophys. Acta 1445, 53–64 - PubMed
-
- Cartier R., Reszka R. (2002) Gene Therapy 9, 157–167 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

