JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis
- PMID: 19638343
- PMCID: PMC2785347
- DOI: 10.1074/jbc.M109.022491
JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis
Abstract
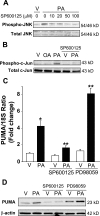
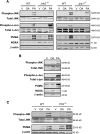
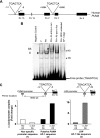
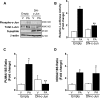
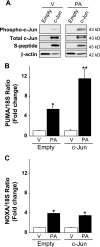
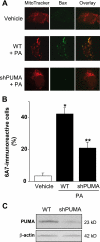
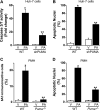
Free fatty acids (FFA) induce hepatocyte lipoapoptosis by a c-Jun N-terminal kinase (JNK)-dependent mechanism. However, the cellular processes by which JNK engages the core apoptotic machinery during lipotoxicity, especially activation of BH3-only proteins, remain incompletely understood. Thus, our aim was to determine whether JNK mediates induction of BH3-only proteins during hepatocyte lipoapoptosis. The saturated FFA palmitate, but not the monounsaturated FFA oleate, induces an increase in PUMA mRNA and protein levels. Palmitate induction of PUMA was JNK1-dependent in primary murine hepatocytes. Palmitate-mediated PUMA expression was inhibited by a dominant negative c-Jun, and direct binding of a phosphorylated c-Jun containing the activator protein 1 complex to the PUMA promoter was identified by electrophoretic mobility shift assay and a chromatin immunoprecipitation assay. Short hairpin RNA-targeted knockdown of PUMA attenuated Bax activation, caspase 3/7 activity, and cell death. Similarly, the genetic deficiency of Puma rendered murine hepatocytes resistant to lipoapoptosis. PUMA expression was also increased in liver biopsy specimens from patients with non-alcoholic steatohepatitis as compared with patients with simple steatosis or controls. Collectively, the data implicate JNK1-dependent PUMA expression as a mechanism contributing to hepatocyte lipoapoptosis.
Figures
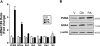

References
-
- Browning J. D., Szczepaniak L. S., Dobbins R., Nuremberg P., Horton J. D., Cohen J. C., Grundy S. M., Hobbs H. H. (2004) Hepatology 40, 1387–1395 - PubMed
-
- Adams L. A., Lymp J. F., St Sauver J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. (2005) Gastroenterology 129, 113–121 - PubMed
-
- Ekstedt M., Franzén L. E., Mathiesen U. L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. (2006) Hepatology 44, 865–873 - PubMed
-
- Ratziu V., Poynard T. (2006) Hepatology 44, 802–805 - PubMed
-
- Bookman I. D., Pham J., Guindi M., Heathcote E. J. (2006) Liver Int. 26, 566–571 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

