Folate rescues lithium-, homocysteine- and Wnt3A-induced vertebrate cardiac anomalies
- PMID: 19638421
- PMCID: PMC2737056
- DOI: 10.1242/dmm.001438
Folate rescues lithium-, homocysteine- and Wnt3A-induced vertebrate cardiac anomalies
Abstract
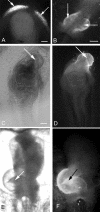
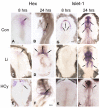
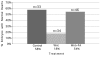
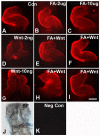
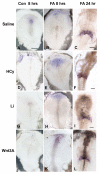
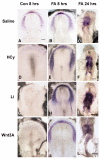
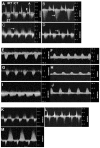
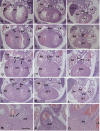
Elevated plasma homocysteine (HCy), which results from folate (folic acid, FA) deficiency, and the mood-stabilizing drug lithium (Li) are both linked to the induction of human congenital heart and neural tube defects. We demonstrated previously that acute administration of Li to pregnant mice on embryonic day (E)6.75 induced cardiac valve defects by potentiating Wnt-beta-catenin signaling. We hypothesized that HCy may similarly induce cardiac defects during gastrulation by targeting the Wnt-beta-catenin pathway. Because dietary FA supplementation protects from neural tube defects, we sought to determine whether FA also protects the embryonic heart from Li- or HCy-induced birth defects and whether the protection occurs by impacting Wnt signaling. Maternal elevation of HCy or Li on E6.75 induced defective heart and placental function on E15.5, as identified non-invasively using echocardiography. This functional analysis of HCy-exposed mouse hearts revealed defects in tricuspid and semilunar valves, together with altered myocardial thickness. A smaller embryo and placental size was observed in the treated groups. FA supplementation ameliorates the observed developmental errors in the Li- or HCy-exposed mouse embryos and normalized heart function. Molecular analysis of gene expression within the avian cardiogenic crescent determined that Li, HCy or Wnt3A suppress Wnt-modulated Hex (also known as Hhex) and Islet-1 (also known as Isl1) expression, and that FA protects from the gene misexpression that is induced by all three factors. Furthermore, myoinositol with FA synergistically enhances the protective effect. Although the specific molecular epigenetic control mechanisms remain to be defined, it appears that Li or HCy induction and FA protection of cardiac defects involve intimate control of the canonical Wnt pathway at a crucial time preceding, and during, early heart organogenesis.
Figures
Similar articles
-
Acute alcohol exposure during mouse gastrulation alters lipid metabolism in placental and heart development: Folate prevention.Birth Defects Res A Clin Mol Teratol. 2016 Sep;106(9):749-60. doi: 10.1002/bdra.23526. Epub 2016 Jun 14. Birth Defects Res A Clin Mol Teratol. 2016. PMID: 27296863 Free PMC article.
-
Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate.Am J Obstet Gynecol. 2010 Jul;203(1):75.e7-75.e15. doi: 10.1016/j.ajog.2010.03.017. Epub 2010 May 10. Am J Obstet Gynecol. 2010. PMID: 20451895
-
Molecular effects of lithium exposure during mouse and chick gastrulation and subsequent valve dysmorphogenesis.Birth Defects Res A Clin Mol Teratol. 2008 Jul;82(7):508-18. doi: 10.1002/bdra.20448. Birth Defects Res A Clin Mol Teratol. 2008. PMID: 18418887
-
Folate protection from congenital heart defects linked with canonical Wnt signaling and epigenetics.Curr Opin Pediatr. 2010 Oct;22(5):561-6. doi: 10.1097/MOP.0b013e32833e2723. Curr Opin Pediatr. 2010. PMID: 20844350 Free PMC article. Review.
-
When should we prescribe high-dose folic acid to prevent congenital heart defects?Curr Opin Cardiol. 2015 Jan;30(1):125-31. doi: 10.1097/HCO.0000000000000124. Curr Opin Cardiol. 2015. PMID: 25389654 Review.
Cited by
-
Acute alcohol exposure during mouse gastrulation alters lipid metabolism in placental and heart development: Folate prevention.Birth Defects Res A Clin Mol Teratol. 2016 Sep;106(9):749-60. doi: 10.1002/bdra.23526. Epub 2016 Jun 14. Birth Defects Res A Clin Mol Teratol. 2016. PMID: 27296863 Free PMC article.
-
Inhibiting MARSs reduces hyperhomocysteinemia-associated neural tube and congenital heart defects.EMBO Mol Med. 2020 Mar 6;12(3):e9469. doi: 10.15252/emmm.201809469. Epub 2020 Jan 31. EMBO Mol Med. 2020. PMID: 32003121 Free PMC article.
-
Autoantibodies against homocysteinylated protein in a mouse model of folate deficiency-induced neural tube defects.Birth Defects Res A Clin Mol Teratol. 2016 Mar;106(3):201-7. doi: 10.1002/bdra.23483. Epub 2016 Feb 22. Birth Defects Res A Clin Mol Teratol. 2016. PMID: 26900104 Free PMC article.
-
Epigenetics and Congenital Heart Diseases.J Cardiovasc Dev Dis. 2022 Jun 9;9(6):185. doi: 10.3390/jcdd9060185. J Cardiovasc Dev Dis. 2022. PMID: 35735814 Free PMC article. Review.
-
The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence?Front Physiol. 2018 Aug 7;9:1045. doi: 10.3389/fphys.2018.01045. eCollection 2018. Front Physiol. 2018. PMID: 30131711 Free PMC article.
References
-
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. (2008). Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development 135, 237–247 - PubMed
-
- Belmaker RH, Agam G, van Calker D, Richards MH, Kofman O. (1998). Behavioral reversal of lithium effects by four inositol isomers correlates perfectly with biochemical effects on the PI cycle. Neuropsychopharmacology 19, 220–232 - PubMed
-
- Boot MJ, Steegers-Theunissen RP, Poelmann RE, van Iperen L, Gittenberger-de Groot AC. (2004). Cardiac outflow tract malformations in chick embryos exposed to homocysteine. Cardiovasc Res. 64, 365–373 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

