Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft
- PMID: 19638451
- PMCID: PMC2728169
- DOI: 10.1158/1535-7163.MCT-09-0304
Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft
Abstract
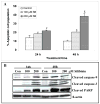
Colorectal cancer is one of the leading causes of cancer-related morbidity and mortality. The use of nontoxic phytochemicals in the prevention and intervention of colorectal cancer has been suggested as an alternative to chemotherapy. Here we assessed the anticancer efficacy of silibinin against advanced colorectal cancer LoVo cells both in vitro and in vivo. Our results showed that silibinin treatment strongly inhibits the growth of LoVo cells (P < 0.05-0.001) and induces apoptotic death (P < 0.01-0.001), which was associated with increased levels of cleaved caspases (3 and 9) and cleaved poly(ADP-ribose) polymerase. Additionally, silibinin caused a strong cell cycle arrest at G(1) phase and a slight but significant G(2)-M-phase arrest at highest concentration (P < 0.01-0.001). Molecular analyses for cell cycle regulators showed that silibinin decreases the level of cyclins (D1, D3, A and B1) and cyclin-dependent kinases (1, 2, 4, and 6) and increases the level of cyclin-dependent kinase inhibitors (p21 and p27). Consistent with these results, silibinin treatment also decreased the phosphorylation of retinoblastoma protein at Ser(780), Ser(795), and Ser(807)/Ser(811) sites without significantly affecting its total level. In animal studies, oral administration of silibinin for 6 weeks (at 100 and 200 mg/kg/d for 5 days/wk) significantly inhibited the growth of LoVo xenograft (P < 0.001) in athymic nude mice without any apparent toxicity. Analyses of xenograft tissue showed that silibinin treatment inhibits proliferation and increases apoptosis along with a strong increase in p27 levels but a decrease in retinoblastoma phosphorylation. Together, these results suggest the potential use of silibinin against advanced human colorectal cancer.
Figures
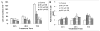




References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Millikan KW, Staren ED, Doolas A. Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am. 1997;77:27–48. - PubMed
-
- Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II–III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–803. - PubMed
-
- Naithani R, Huma LC, Moriarty RM, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044–71. - PubMed
-
- Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Front Biosci. 2007;12:2309–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

