The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity
- PMID: 19638587
- PMCID: PMC2875168
- DOI: 10.1158/0008-5472.CAN-09-0301
The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity
Abstract
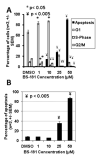
Normal progression through the cell cycle requires the sequential action of cyclin-dependent kinases CDK1, CDK2, CDK4, and CDK6. Direct or indirect deregulation of CDK activity is a feature of almost all cancers and has led to the development of CDK inhibitors as anticancer agents. The CDK-activating kinase (CAK) plays a critical role in regulating cell cycle by mediating the activating phosphorylation of CDK1, CDK2, CDK4, and CDK6. As such, CDK7, which also regulates transcription as part of the TFIIH basal transcription factor, is an attractive target for the development of anticancer drugs. Computer modeling of the CDK7 structure was used to design potential potent CDK7 inhibitors. Here, we show that a pyrazolo[1,5-a]pyrimidine-derived compound, BS-181, inhibited CAK activity with an IC(50) of 21 nmol/L. Testing of other CDKs as well as another 69 kinases showed that BS-181 only inhibited CDK2 at concentrations lower than 1 micromol/L, with CDK2 being inhibited 35-fold less potently (IC(50) 880 nmol/L) than CDK7. In MCF-7 cells, BS-181 inhibited the phosphorylation of CDK7 substrates, promoted cell cycle arrest and apoptosis to inhibit the growth of cancer cell lines, and showed antitumor effects in vivo. The drug was stable in vivo with a plasma elimination half-life in mice of 405 minutes after i.p. administration of 10 mg/kg. The same dose of drug inhibited the growth of MCF-7 human xenografts in nude mice. BS-181 therefore provides the first example of a potent and selective CDK7 inhibitor with potential as an anticancer agent.
Figures



References
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. - PubMed
-
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. - PubMed
-
- Fisher RP. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci. 2005;118:5171–80. - PubMed
-
- Harper JW, Elledge SJ. The role of Cdk7 in CAK function, a retro-retrospective. Genes Dev. 1998;12:285–9. - PubMed
-
- Lolli G, Johnson LN. CAK-Cyclin-dependent Activating Kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle. 2005;4:572–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

