Inhibition of superoxide generation upon T-cell receptor engagement rescues Mart-1(27-35)-reactive T cells from activation-induced cell death
- PMID: 19638595
- PMCID: PMC2719828
- DOI: 10.1158/0008-5472.CAN-09-1176
Inhibition of superoxide generation upon T-cell receptor engagement rescues Mart-1(27-35)-reactive T cells from activation-induced cell death
Abstract
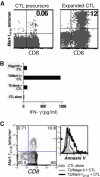
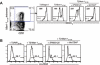
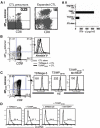
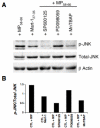
Cytotoxic T lymphocytes (CTL) may undergo massive expansion upon appropriate antigenic stimulation. Homeostasis is maintained by a subsequent "contraction" of these cells. Activation-induced cell death (AICD) and programmed cell death prevent the untoward side effects, arising from excessive numbers and prolonged persistence of activated CTL, that occur upon uncontrolled and/or continued expansion. However, effector cell persistence has been identified as a hallmark of successful T-cell-mediated adoptive immunotherapy. Thus, prevention of AICD may be critical to achieve more successful clinical results. We have previously shown that treatment with the c-Jun NH(2)-terminal kinase (JNK) inhibitor SP600125 protects human melanoma epitope Mart-1(27-35)-reactive CTL from apoptotic death upon their reencounter with cognate antigen. However, inhibition of JNK also interferes with the functional ability of the CTL to secrete IFN-gamma. Here, we show that reactive oxygen species (ROS) inhibitors, such as the superoxide dismutase mimetic Mn (III) tetrakis (5, 10, 15, 20-benzoic acid) porphyrin (MnTBAP), efficiently protected Mart-1(27-35)-reactive primary CTL from AICD without impairing their functional capability. MnTBAP prevented the increase in intracellular ROS, mitochondrial membrane collapse, and DNA fragmentation observed in control-treated cells upon cognate antigen encounter. Furthermore, the mechanism of AICD prevention in primary CTL included blockade of JNK activation. Finally, tumor-reactive in vitro expanded tumor infiltrating lymphocytes, which are used clinically in cancer immunotherapy, also benefit from MnTBAP-mediated antioxidant treatment. Thus, modulation of the redox pathway might improve CTL persistence and lead to better clinical results for T cell-based immunotherapies.
Figures


Comment in
- Immunotherapy. 2009 Nov;1(6):921-4
References
-
- Jaattela M. Programmed cell death: many ways for cells to die decently. Ann Med. 2002;34:480–8. - PubMed
-
- Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–9. - PubMed
-
- Whiteside TL. Apoptosis of immune cells in the tumor microenvironment and peripheral circulation of patients with cancer: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A46–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

