Generating differentially targeted amyloid-beta specific intrabodies as a passive vaccination strategy for Alzheimer's disease
- PMID: 19638957
- PMCID: PMC2788047
- DOI: 10.1038/mt.2009.174
Generating differentially targeted amyloid-beta specific intrabodies as a passive vaccination strategy for Alzheimer's disease
Abstract
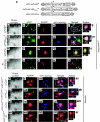
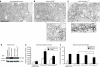
Amyloid-beta (A beta) has been identified as a key component in Alzheimer's disease (AD). Significant in vitro and human pathological data suggest that intraneuronal accumulation of A beta peptides plays an early role in the neurodegenerative cascade. We hypothesized that targeting an antibody-based therapeutic to specifically abrogate intracellular A beta accumulation could prevent or slow disease onset. A beta 42-specific intracellular antibodies (intrabodies) with and without an intracellular trafficking signal were engineered from a previously characterized single-chain variable fragment (scFv) antibody. The intrabodies, one with an endoplasmic reticulum (ER) targeting signal and one devoid of a targeting sequence, were assessed in cells harboring a doxycycline (Dox)-regulated mutant human amyloid precursor protein Swedish mutant (hAPP(swe)) transcription unit for their abilities to prevent A beta peptide egress. Adeno-associated virus (AAV) vectors expressing the engineered intrabodies were administered to young adult 3xTg-AD mice, a model that develops amyloid and Tau pathologies, prior to the initial appearance of intraneuronal A beta. Chronic expression of the ER-targeted intrabody (IB) led to partial clearance of A beta 42 deposits and interestingly, in reduced staining for a pathologic phospho-Tau epitope (Thr231). This approach may provide insights into the functional relevance of intraneuronal A beta accumulation in early AD and potentially lead to the development of new therapeutics.
Figures




Similar articles
-
Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice.J Neurosci. 2006 Nov 15;26(46):11923-8. doi: 10.1523/JNEUROSCI.2795-06.2006. J Neurosci. 2006. PMID: 17108166 Free PMC article.
-
Targeting tauopathy with engineered tau-degrading intrabodies.Mol Neurodegener. 2019 Oct 22;14(1):38. doi: 10.1186/s13024-019-0340-6. Mol Neurodegener. 2019. PMID: 31640765 Free PMC article.
-
Intramuscular delivery of p75NTR ectodomain by an AAV vector attenuates cognitive deficits and Alzheimer's disease-like pathologies in APP/PS1 transgenic mice.J Neurochem. 2016 Jul;138(1):163-73. doi: 10.1111/jnc.13616. Epub 2016 Jun 6. J Neurochem. 2016. PMID: 26991827
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Vaccination as a therapeutic approach to Alzheimer's disease.Mt Sinai J Med. 2010 Jan-Feb;77(1):17-31. doi: 10.1002/msj.20156. Mt Sinai J Med. 2010. PMID: 20101719 Free PMC article. Review.
Cited by
-
Antibodies inside of a cell can change its outside: Can intrabodies provide a new therapeutic paradigm?Comput Struct Biotechnol J. 2016 Jul 31;14:304-8. doi: 10.1016/j.csbj.2016.07.003. eCollection 2016. Comput Struct Biotechnol J. 2016. PMID: 27570612 Free PMC article. Review.
-
Early oligodendrocyte/myelin pathology in Alzheimer's disease mice constitutes a novel therapeutic target.Am J Pathol. 2010 Sep;177(3):1422-35. doi: 10.2353/ajpath.2010.100087. Epub 2010 Aug 9. Am J Pathol. 2010. PMID: 20696774 Free PMC article.
-
AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System.Front Neurol. 2022 Apr 12;13:870799. doi: 10.3389/fneur.2022.870799. eCollection 2022. Front Neurol. 2022. PMID: 35493843 Free PMC article. Review.
-
Amyloid-β oligomer specificity mediated by the IgM isotype--implications for a specific protective mechanism exerted by endogenous auto-antibodies.PLoS One. 2010 Nov 10;5(11):e13928. doi: 10.1371/journal.pone.0013928. PLoS One. 2010. PMID: 21085663 Free PMC article.
-
Engineered Extracellular Vesicles/Exosomes as a New Tool against Neurodegenerative Diseases.Pharmaceutics. 2020 Jun 9;12(6):529. doi: 10.3390/pharmaceutics12060529. Pharmaceutics. 2020. PMID: 32526949 Free PMC article. Review.
References
-
- Chui DH, Dobo E, Makifuchi T, Akiyama H, Kawakatsu S, Petit A, et al. Apoptotic neurons in Alzheimer's disease frequently show intracellular Abeta42 labeling. J Alzheimers Dis. 2001;3:231–239. - PubMed
-
- Kienlen-Campard P, Miolet S, Tasiaux B., and , Octave JN. Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem. 2002;277:15666–15670. - PubMed
-
- LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC., and , Jay G. The Alzheimer's A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. - PubMed
-
- Takeda K, Araki W., and , Tabira T. Enhanced generation of intracellular Abeta42 amyloid peptide by mutation of presenilins PS1 and PS2. Eur J Neurosci. 2004;19:258–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

