Peripheral neural cell sensitivity to mTHPC-mediated photodynamic therapy in a 3D in vitro model
- PMID: 19638975
- PMCID: PMC2736832
- DOI: 10.1038/sj.bjc.6605197
Peripheral neural cell sensitivity to mTHPC-mediated photodynamic therapy in a 3D in vitro model
Abstract
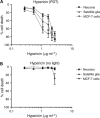
Background: The effect of photodynamic therapy (PDT) on neural cells is important when tumours are within or adjacent to the nervous system. The purpose of this study was to investigate PDT using the photosensitiser, meta-tetrahydroxyphenyl chlorin (mTHPC), on rat neurons and satellite glia, compared with human adenocarcinoma cells (MCF-7).
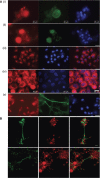
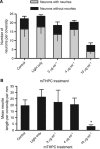
Methods: Fluorescence microscopy confirmed that mTHPC was incorporated into all three cell types. Sensitivity of cells exposed to mTHPC-PDT (0-10 microg ml(-1)) was determined in a novel 3-dimensional collagen gel culture system. Cell death was quantified using propidium iodide and cell types were distinguished using immunocytochemistry. In some cases, neuron survival was confirmed by measuring subsequent neurite growth in monolayer culture.
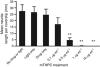
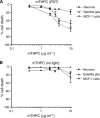
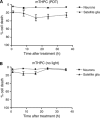
Results: MCF-7s and satellite glia were significantly more sensitive to PDT than neurons. Importantly, 4 microg ml(-1) mTHPC-PDT caused no significant neuron death compared with untreated controls but was sufficient to elicit substantial cell death in the other cell types. Initially, treatment reduced neurite length; neurons then extended neurites equivalent to those of untreated controls. The protocol was validated using hypericin (0-3 microg ml(-1)), which caused neuron death equivalent to other cell types.
Conclusion: Neurons in culture can survive mTHPC-PDT under conditions sufficient to kill tumour cells and other nervous system cells.
Figures
Similar articles
-
Inhibition of specific cellular antioxidant pathways increases the sensitivity of neurons to meta-tetrahydroxyphenyl chlorin-mediated photodynamic therapy in a 3D co-culture model.Photochem Photobiol. 2012 Nov-Dec;88(6):1539-45. doi: 10.1111/j.1751-1097.2012.01185.x. Epub 2012 Jul 9. Photochem Photobiol. 2012. PMID: 22671577
-
Schedule-dependent interaction between Doxorubicin and mTHPC-mediated photodynamic therapy in murine hepatoma in vitro and in vivo.Cancer Chemother Pharmacol. 2006 Jan;57(1):65-72. doi: 10.1007/s00280-005-0006-7. Epub 2005 Nov 5. Cancer Chemother Pharmacol. 2006. PMID: 16001168
-
Effective treatment of liver metastases with photodynamic therapy, using the second-generation photosensitizer meta-tetra(hydroxyphenyl)chlorin (mTHPC), in a rat model.Br J Cancer. 1999 Oct;81(4):600-8. doi: 10.1038/sj.bjc.6690736. Br J Cancer. 1999. PMID: 10574244 Free PMC article.
-
Experimental evaluation of possible side effects of intra-operative photodynamic therapy on rabbit blood vessels and nerves.Lasers Surg Med. 2003;33(4):247-55. doi: 10.1002/lsm.10220. Lasers Surg Med. 2003. PMID: 14571449
-
mTHPC mediated, systemic photodynamic therapy (PDT) for nonmelanoma skin cancers: Case and literature review.Lasers Surg Med. 2015 Dec;47(10):779-87. doi: 10.1002/lsm.22429. Epub 2015 Oct 14. Lasers Surg Med. 2015. PMID: 26462858 Review.
Cited by
-
Could clinical photochemical internalisation be optimised to avoid neuronal toxicity?Int J Pharm. 2017 Aug 7;528(1-2):133-143. doi: 10.1016/j.ijpharm.2017.05.071. Epub 2017 Jun 1. Int J Pharm. 2017. PMID: 28579544 Free PMC article.
-
Treatment of 3D In Vitro Tumoroids of Ovarian Cancer Using Photochemical Internalisation as a Drug Delivery Method.Biomedicines. 2023 Feb 15;11(2):572. doi: 10.3390/biomedicines11020572. Biomedicines. 2023. PMID: 36831108 Free PMC article.
-
Toward a 3D cellular model for studying in vitro the outcome of photodynamic treatments: accounting for the effects of tissue complexity.Tissue Eng Part A. 2013 Aug;19(15-16):1665-74. doi: 10.1089/ten.TEA.2012.0661. Epub 2013 Apr 19. Tissue Eng Part A. 2013. PMID: 23442191 Free PMC article.
-
Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro.FASEB J. 2014 Apr;28(4):1634-43. doi: 10.1096/fj.13-243980. Epub 2013 Dec 18. FASEB J. 2014. PMID: 24352035 Free PMC article.
-
The Effect of Hypothermic and Cryogenic Preservation on Engineered Neural Tissue.Tissue Eng Part C Methods. 2017 Oct;23(10):575-582. doi: 10.1089/ten.TEC.2017.0244. Tissue Eng Part C Methods. 2017. PMID: 28877649 Free PMC article.
References
-
- Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ (2007) ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13: 2863–2870 - PubMed
-
- Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ (2008) Laminin activates NF-kappa B in Schwann cells to enhance neurite outgrowth. Neurosci Lett 439: 42–46 - PubMed
-
- Betz CS, Jager HR, Brookes JA, Richards R, Leunig A, Hopper C (2007) Interstitial photodynamic therapy for a symptom-targeted treatment of complex vascular malformations in the head and neck region. Lasers Surg Med 39: 571–582 - PubMed
-
- Brown RA, Phillips JB (2007) Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol 262: 75–150 - PubMed
-
- Castro C, Kuffler DP (2006) Membrane-bound CSPG mediates growth cone outgrowth and substrate specificity by Schwann cell contact with the DRG neuron cell body and not via growth cone contact. Exp Neurol 200: 19–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

