A Phase 3, Randomized, Placebo-controlled Trial of Filgrastim in Patients with Haematological Malignancies Undergoing Matched-related Allogeneic Bone Marrow Transplantation
- PMID: 19639030
- PMCID: PMC2710993
- DOI: 10.1111/j.1753-5174.2008.00013.x
A Phase 3, Randomized, Placebo-controlled Trial of Filgrastim in Patients with Haematological Malignancies Undergoing Matched-related Allogeneic Bone Marrow Transplantation
Abstract
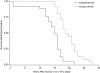
INTRODUCTION: Recombinant granulocyte colony-stimulating factor (G-CSF) may aid engraftment post high-dose chemo-/radiotherapy in patients with haematological malignancies undergoing allogeneic bone marrow transplantation (BMT); however, the effects of G-CSF on graft-versus-host disease (GvHD), relapse, and survival are not well defined. METHODS: In this double-blind, randomized, placebo-controlled, multicentre, phase 3 study, the effects of the G-CSF Filgrastim on neutrophil and platelet recovery, and on clinical outcomes were evaluated. Patients (12-55 years) receiving an allogeneic BMT for a haematological malignancy were randomized to receive Filgrastim 5 microg/kg or placebo. Study treatment was continued until patients achieved an absolute neutrophil count (ANC) >/=0.5 x 10(9)/L, or until day 42. RESULTS: Fifty-one patients (Filgrastim, N = 25; placebo, N = 26) were evaluable. Patients treated with Filgrastim had significantly faster engraftment with ANC >/=0.5 x 10(9)/L being achieved after a median (range) of 15.0 (1.0-22.0) days vs. 19.0 (15.0-28.0) days for placebo (P< 0.0001). The incidence of GvHD was comparable for both groups. During the limited follow-up (2 years), Filgrastim had no adverse effect on mortality and possibly reduced the rate of relapse.
Figures
References
-
- Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with Chronic myeloid leukaemia before allogeneic Blood or Marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow transplantation. Lancet. 1998;352:1087–92. - PubMed
-
- Schriber JR, Chao NJ, Long GD, Negrin RS, Tierney DK, Kusnierz-Glaz C, et al. Granulocyte colony-stimulating factor after allogeneic bone marrow transplantation. Blood. 1994;84:1680–4. - PubMed
-
- Berger C, Bertz H, Schmoor C, Behringer D, Potthoff K, Mertelsmann R, et al. Influence of recombinant human granulocyte colony-stimulating factor (Filgrastim) on hematopoietic recovery and outcome following allogeneic bone marrow transplantation (BMT) from volunteer unrelated donors. Bone Marrow Transplant. 1999;23:983–90. - PMC - PubMed
-
- Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, et al. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96:80–5. - PubMed
-
- Przepiorka D, Smith TL, Folloder J, Anderlini P, Chan KW, Korbling M, et al. Controlled trial of filgrastim for acceleration of neutrophil recovery after allogeneic blood stem cell transplantation from human leukocyte antigen-matched related donors. Blood. 2001;97:3405–10. - PubMed
LinkOut - more resources
Full Text Sources