Utility of Pretreatment Bilirubin Level and UGT1A1 Polymorphisms in Multivariate Predictive Models of Neutropenia Associated with Irinotecan Treatment in Previously Untreated Patients with Colorectal Cancer
- PMID: 19639031
- PMCID: PMC2710994
- DOI: 10.1111/j.1753-5174.2008.00014.x
Utility of Pretreatment Bilirubin Level and UGT1A1 Polymorphisms in Multivariate Predictive Models of Neutropenia Associated with Irinotecan Treatment in Previously Untreated Patients with Colorectal Cancer
Abstract
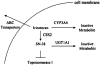
PURPOSE: Statistical models for predicting hematologic toxicity were evaluated based on UGT1A1 polymorphisms and baseline serum bilirubin. METHODS: Blood DNA samples were collected from 113 patients with untreated metastatic colorectal cancer receiving irinotecan (FOLFIRI, n = 36; mIFL, n = 41; CapeIRI, n = 36). The primary endpoint was absolute neutrophil count nadir during first treatment cycle. Linear regression models, with increased R(2) implying important additional predictive power, sequentially added age, sex, baseline bilirubin level, and UGT1A1 genotype. RESULTS: All models demonstrated low R(2), suggesting unaccounted variables. UGT1A1 genotype added approximately 8-9% during cycle 1 and from approximately 7% [mIFL regimen] to 26% [CapeIRI regimen] after cycle 1. Correlation between genotype and overall ANC nadir without regard to treatment was low (R = -0.201, P = 0.035). Patients with genotype 7/7 may have increased risk for severe neutropenia, but data are insufficient to characterize this. Contribution of baseline bilirubin level was negligible. CONCLUSIONS: Ability of UGT1A1 or baseline bilirubin to predict neutropenia is low and depends on regimen.
Figures

References
-
- Tan BR, McLeod HL. Pharmacogenetic influences on treatment response and toxicity in colorectal cancer. Semin Oncol. 2005;32:113–9. - PubMed
-
- Goldberg RM. Therapy for metastatic colorectal cancer. Oncologist. 2006;11:981–7. - PubMed
-
- Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–61. - PubMed
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–37. - PubMed
-
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–14. - PubMed
LinkOut - more resources
Full Text Sources
