Introduction to adenosine receptors as therapeutic targets
- PMID: 19639277
- PMCID: PMC3415694
- DOI: 10.1007/978-3-540-89615-9_1
Introduction to adenosine receptors as therapeutic targets
Abstract
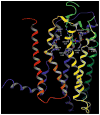
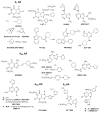
Adenosine acts as a cytoprotective modulator in response to stress to an organ or tissue. Although short-lived in the circulation, it can activate four subtypes of G protein-coupled adenosine receptors (ARs): A(1), A(2A), A(2B), and A(3). The alkylxanthines caffeine and theophylline are the prototypical antagonists of ARs, and their stimulant actions occur primarily through this mechanism. For each of the four AR subtypes, selective agonists and antagonists have been introduced and used to develop new therapeutic drug concepts. ARs are notable among the GPCR family in the number and variety of agonist therapeutic candidates that have been proposed. The selective and potent synthetic AR agonists, which are typically much longer lasting in the body than adenosine, have potential therapeutic applications based on their anti-inflammatory (A(2A) and A(3)), cardioprotective (preconditioning by A(1) and A(3) and postconditioning by A(2B)), cerebroprotective (A(1) and A(3)), and antinociceptive (A(1)) properties. Potent and selective AR antagonists display therapeutic potential as kidney protective (A(1)), antifibrotic (A(2A)), neuroprotective (A(2A)), and antiglaucoma (A(3)) agents. AR agonists for cardiac imaging and positron-emitting AR antagonists are in development for diagnostic applications. Allosteric modulators of A(1) and A(3) ARs have been described. In addition to the use of selective agonists/antagonists as pharmacological tools, mouse strains in which an AR has been genetically deleted have aided in developing novel drug concepts based on the modulation of ARs.
Figures


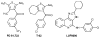
References
-
- Akaiwa K, Akashi H, Harada H, Sakashita H, Hiromatsu S, Kano T, Aoyagi S. Moderate cerebral venous congestion induces rapid cerebral protection via adenosine A1 receptor activation. Brain Res. 2006;1122:47–55. - PubMed
-
- Auchampach JA, Jin X, Moore J, Wan TC, Kreckler LM, Ge ZD, Narayanan J, Whalley E, Kiesman W, Ticho B, Smits G, Gross GJ. Comparison of three different A1 adenosine receptor antagonists on infarct size and multiple cycle ischemic preconditioning in anesthetized dogs. J Pharmacol Exp Ther. 2004;308:846–856. - PubMed
-
- Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. - PubMed
-
- Baraldi PG, Tabrizi MA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev. 2008;108:238–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

