Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering
- PMID: 19639281
- PMCID: PMC3413728
- DOI: 10.1007/978-3-540-89615-9_5
Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering
Abstract
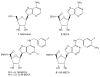
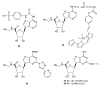
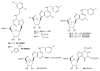
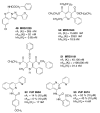
A(3) adenosine receptor (A(3)AR) ligands have been modified to optimize their interaction with the A(3)AR. Most of these modifications have been made to the N(6) and C2 positions of adenine as well as the ribose moiety, and using a combination of these substitutions leads to the most efficacious, selective, and potent ligands. A(3)AR agonists such as IB-MECA and Cl-IB-MECA are now advancing into Phase II clinical trials for treatments targeting diseases such as cancer, arthritis, and psoriasis. Also, a wide number of compounds exerting high potency and selectivity in antagonizing the human (h)A(3)AR have been discovered. These molecules are generally characterized by a notable structural diversity, taking into account that aromatic nitrogen-containing monocyclic (thiazoles and thiadiazoles), bicyclic (isoquinoline, quinozalines, (aza)adenines), tricyclic systems (pyrazoloquinolines, triazoloquinoxalines, pyrazolotriazolopyrimidines, triazolopurines, tricyclic xanthines) and nucleoside derivatives have been identified as potent and selective A(3)AR antagonists. Probably due to the "enigmatic" physiological role of A(3)AR, whose activation may produce opposite effects (for example, concerning tissue protection in inflammatory and cancer cells) and may produce effects that are species dependent, only a few molecules have reached preclinical investigation. Indeed, the most advanced A(3)AR antagonists remain in preclinical testing. Among the antagonists described above, compound OT-7999 is expected to enter clinical trials for the treatment of glaucoma, while several thiazole derivatives are in development as antiallergic, antiasthmatic and/or antiinflammatory drugs.
Figures

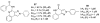

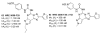
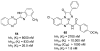
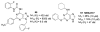



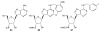
References
-
- Baharav E, Bar-Yehuda S, Madi L, Silberman D, Rath-Wolfson L, Halpren M, Ochaion A, Weinberger A, Fishman P. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol. 2005;32:469–476. - PubMed
-
- Baraldi PG, Cacciari B, de las Infantas MJ Pineda, Romagnoli R, Spalluto G, Volpini R, Costanzi S, Vittori S, Cristalli G, Melman N, Park K-S, Ji X-d, Jacobson KA. Synthesis and biological activity of a new series of N6-arylcarbamoyl-, 2-(ar)alkynyl-N6-arylcarbamoyl, and N6-carboxamido-derivatives of adenosine-5′ -N-ethyluronamide (NECA) as A1 and A3 adenosine receptor agonists. J Med Chem. 1998;41:3174–3185. - PMC - PubMed
-
- Baraldi PG, Cacciari B, Borea PA, Varani K, Pastorin G, Da Ros T, Spalluto G. Pyrazolotriazolo-pyrimidine derivatives as adenosine receptor antagonists: a possible template for adenosine receptor subtypes? Curr Pharm Design. 2002a;8:99–110. - PubMed
-
- Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Monopoli A, Ongini E, Varani K, Borea PA. 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as A2A adenosine receptor antagonists: a study on the importance of modifications at the side chain on the activity and solubility. J Med Chem. 2002b;45:115–126. - PubMed
-
- Baraldi PG, Tabrizi MA, Fruttarolo F, Bovero A, Avitabile B, Preti D, Romagnoli R, Merighi S, Gessi S, Varani K, Borea PA. Recent developments in the field of A3 adenosine receptor antagonists. Drug Dev Res. 2003a;58:315–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

