Meiotic behavior of aneuploid chromatin in mouse models of Down syndrome
- PMID: 19639331
- PMCID: PMC2848991
- DOI: 10.1007/s00412-009-0230-8
Meiotic behavior of aneuploid chromatin in mouse models of Down syndrome
Abstract
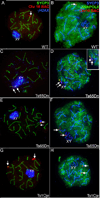
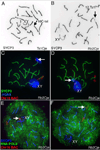
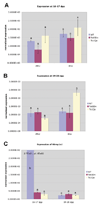
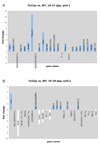
Aneuploidy, which leads to unpaired chromosomal axes during meiosis, is frequently accompanied by infertility. We previously showed, using three mouse models of Down syndrome, that it is an extra chromosome, but not extra gene dose, that is associated with male infertility and virtual absence of post-meiotic gem cells. Here, we test the hypothesis that aneuploid segments are differentially modified and expressed during meiosis, depending on whether they are present as an extra chromosome or not. In all three models examined, the trisomic region lacks a pairing partner, but in one case, spermatocytes have an extra (and unpaired) chromosome, while the two other models involve translocation of the trisomic region rather than an extra chromosome. An extra unpaired chromosome was always modified by phosphorylation of histone H2AX and lacked RNA PolII. But in the case of trisomic regions attached to a paired chromosome, assembly of these protein modifications was affected by the position of a trisomic region relative to a centromere and the physical extent of the unpaired chromatin. Analysis of gene expression in testes revealed that extra copy number alone was not sufficient for meiotic upregulation of genes in the trisomic interval. Additionally and unexpectedly, presence of meiotic gene silencing chromatin modifications was not sufficient for downregulation of genes in unpaired trisomic chromatin. Thus, the meiotic chromatin modifications that are cytologically visible are unlikely to be directly involved in sterility versus fertility of DS models. Finally, the presence of an extra unpaired chromosome, but not the presence of extra (trisomic) genes, caused global deregulation of transcription in spermatocytes. These results reveal mechanisms by which an extra chromosome, but not trisomic gene dose, impact on meiotic progress and infertility.
Figures
References
-
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J Mol Endocrinol. 2002;29:23–39. - PubMed
-
- Cheng Z, Tu C, Rodriguez L, Chen TH, Dvorak MM, Margeta M, Gassmann M, Bettler B, Shoback D, Chang W. Type B gamma-aminobutyric acid receptors modulate the function of the extracellular Ca2+sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992. - PubMed
-
- Cui X, Hwang JT, Qiu J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics. 2005;6:59–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

