Squaraine rotaxanes with boat conformation macrocycles
- PMID: 19639940
- PMCID: PMC2847772
- DOI: 10.1021/jo901298n
Squaraine rotaxanes with boat conformation macrocycles
Abstract
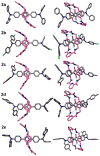
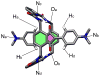
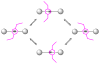
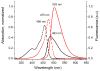
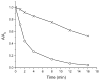
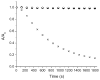
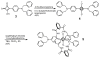
Mechanical encapsulation of fluorescent, deep-red bis(anilino)squaraine dyes inside Leigh-type tetralactam macrocycles produces interlocked squaraine rotaxanes. The surrounding macrocycles are flexible and undergo rapid exchange of chair and boat conformations in solution. A series of X-ray crystal structures show how the rotaxane co-conformational exchange process involves simultaneous lateral oscillation of the macrocycle about the center of the encapsulated squaraine thread. Rotaxane macrocycles with 1,4-phenylene sidewalls and 2,6-pyridine dicarboxamide bridging units are more likely to adopt boat conformations in the solid state than analogous squaraine rotaxane systems with isophthalamide-containing macrocycles. A truncated squaraine dye, with a secondary amine attached directly to the central C(4)O(2) core, is less electrophilic than the extended bis(anilino)squaraine analogue, but it is still susceptible to chemical and photochemical bleaching. Its stability is greatly enhanced when it is encapsulated as an interlocked squaraine rotaxane. An X-ray crystal structure of this truncated squaraine rotaxane shows the macrocycle in a boat conformation, and NMR studies indicate that the boat is maintained in solution. Encapsulation as a rotaxane increases the dye's brightness by a factor of 6. The encapsulation process appears to constrain the dye and reduce deformation of the chromophore from planarity. This study shows how mechanical encapsulation as a rotaxane can be used as a rational design parameter to fine-tune the chemical and photochemical properties of squaraine dyes.
Figures
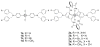

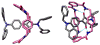
References
-
- Rescifina A, Zagni C, Iannazzo D, Merino P. Curr Org Chem. 2009;13:448–481.
- Kay RE, Leigh DA. Pure Appl Chem. 2008;80:17–29.
- Griffiths KE, Stoddart JF. Pure Appl Chem. 2008;80:485–506.
- Kay ER, Leigh DA, Zerbetto F. Angew Chem Int Ed. 2007;46:72–191. - PubMed
- Loeb SJ. Chem Soc Rev. 2007;36:226–235. - PubMed
- Tian H, Wang QC. Chem Soc Rev. 2006;35:361–374. - PubMed
- Schalley CA, Weilandt T, Bruggemann J, Vögtle F. Top Curr Chem. 2005;248:141–200.
-
- Betancourt JE, Martin-Hidalgo M, Gubala V, Rivera JM. J Am Chem Soc. 2009;131:3186–3188. - PMC - PubMed
- Zhu SS, Nieger M, Daniels J, Felder T, Kossev I, Schmidt T, Sokolowski M, Vogtle F, Schalley CA. Chem Eur J. 2009;15:5040–5046. - PubMed
- Pons M, Millet O. Prog Nucl Magn Reson Spectrosc. 2001;38:267–324.
-
- Leigh DA, Murphy A, Smart JP, Slawin AMZ. Angew Chem Int Ed. 1997;36:728–732.
- Gatti FG, Leigh DA, Nepogodiev SA, Slawin AMZ, Teat SJ, Wong JKY. J Am Chem Soc. 2001;123:5983–5989. - PubMed
- Lane AS, Leigh DA, Murphy A. J Am Chem Soc. 1997;119:11092–11093.
- Clegg W, Gimenez-Saiz C, Leigh DA, Murphy A, Slawin AMZ, Teat SJ. J Am Chem Soc. 1999;121:4124–4129.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

