Detailed evaluation of the geometric and electronic structures of one-electron oxidized group 10 (Ni, Pd, and Pt) metal(II)-(disalicylidene)diamine complexes
- PMID: 19639970
- PMCID: PMC2778000
- DOI: 10.1021/ic901003q
Detailed evaluation of the geometric and electronic structures of one-electron oxidized group 10 (Ni, Pd, and Pt) metal(II)-(disalicylidene)diamine complexes
Abstract
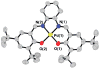
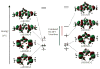
The geometric and electronic structures of a series of one-electron oxidized group 10 metal salens (Ni, Pd, Pt) have been investigated in solution and in the solid state. Ni (1) and Pd (2) complexes of the tetradentate salen ligand N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine (H(2)Salcn) have been examined along with the Pt (3) complex of the salen ligand N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-ethylenediamine (H(2)Salen). All three oxidized compounds exist as ligand radical species in solution and in the solid state. The solid state structures of [1](+) and [3](+) exhibit a symmetric coordination sphere contraction relative to the neutral forms. By contrast, the coordination sphere of the Pd derivative [2](+) exhibits a pronounced asymmetry in the solid state. In solution, the oxidized derivatives display intense low-energy NIR transitions consistent with their classification as ligand radical compounds. Interestingly, the degree of communication between the phenolate moieties depends strongly on the central metal ion, within the Ni, Pd, and Pt series. Electrochemical measurements and UV-vis-NIR spectroscopy, in conjunction with density functional theory calculations provide insights into the degree of delocalization of the one-electron hole in these systems. The Pd complex [2](+) is the least delocalized and is best described as a borderline Class II/III intervalence complex based on the Robin-Day classification system. The Ni [1](+) and Pt [3](+) analogues are Class III (fully delocalized) intervalence compounds. Delocalization is dependent on the electronic coupling between the redox-active phenolate ligands, mediated by overlap between the formally filled metal d(xz) orbital and the appropriate ligand molecular orbital. The degree of coupling increases in the order Pd < Ni < Pt for the one-electron oxidized group 10 metal salens.
Figures
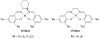






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

