Regressive evolution in Astyanax cavefish
- PMID: 19640230
- PMCID: PMC3594788
- DOI: 10.1146/annurev-genet-102108-134216
Regressive evolution in Astyanax cavefish
Abstract
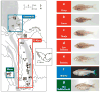
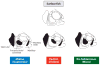
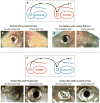
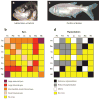
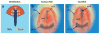
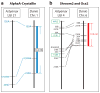
A diverse group of animals, including members of most major phyla, have adapted to life in the perpetual darkness of caves. These animals are united by the convergence of two regressive phenotypes, loss of eyes and pigmentation. The mechanisms of regressive evolution are poorly understood. The teleost Astyanax mexicanus is of special significance in studies of regressive evolution in cave animals. This species includes an ancestral surface dwelling form and many con-specific cave-dwelling forms, some of which have evolved their recessive phenotypes independently. Recent advances in Astyanax development and genetics have provided new information about how eyes and pigment are lost during cavefish evolution; namely, they have revealed some of the molecular and cellular mechanisms involved in trait modification, the number and identity of the underlying genes and mutations, the molecular basis of parallel evolution, and the evolutionary forces driving adaptation to the cave environment.
Figures




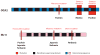
References
-
- Alunni A, Menuet A, Candal E, Pénigault J-B, Jeffery WR, Rétaux S. Developmental mechanisms for retinal degeneration in the blind cavefish Astyanax mexicanus. J Comp Neurol. 2007;505:221–33. - PubMed
-
- Alvarez J. Revisión del generero Anoptichthys con descriptión de una especies nueva (Pisc. Characidae) An Esc Nac Cien Biol Mexico. 1946;4:263–82.
-
- Alvarez J. Descriptión de Anoptichthys hubbsi caracinído ciego de La Cueva de Los Sabinos, S. L. P. Rev Soc Mexicana Hist Nat. 1947;8:215–19.
-
- Behrens M, Wilkens H, Schmale H. Cloning of the αA-crystallin genes of the blind cave form and the epigean form of Astyanax fasciatus: a comparative analysis of structure, expression and evolutionary conservation. Gene. 1998;216:319–26. - PubMed
-
- Borowsky R. Restoring sight in blind cavefish. Curr Biol. 2008;18:R23–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

