Role of KCNMA1 gene in breast cancer invasion and metastasis to brain
- PMID: 19640305
- PMCID: PMC2727533
- DOI: 10.1186/1471-2407-9-258
Role of KCNMA1 gene in breast cancer invasion and metastasis to brain
Abstract
Background: The prognosis for patients with breast tumor metastases to brain is extremely poor. Identification of prognostic molecular markers of the metastatic process is critical for designing therapeutic modalities for reducing the occurrence of metastasis. Although ubiquitously present in most human organs, large-conductance calcium- and voltage-activated potassium channel (BKCa) channels are significantly upregulated in breast cancer cells. In this study we investigated the role of KCNMA1 gene that encodes for the pore-forming alpha-subunit of BKCa channels in breast cancer metastasis and invasion.
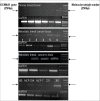
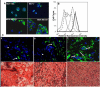
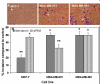
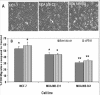
Methods: We performed Global exon array to study the expression of KCNMA1 in metastatic breast cancer to brain, compared its expression in primary breast cancer and breast cancers metastatic to other organs, and validated the findings by RT-PCR. Immunohistochemistry was performed to study the expression and localization of BKCa channel protein in primary and metastatic breast cancer tissues and breast cancer cell lines. We performed matrigel invasion, transendothelial migration and membrane potential assays in established lines of normal breast cells (MCF-10A), non-metastatic breast cancer (MCF-7), non-brain metastatic breast cancer cells (MDA-MB-231), and brain-specific metastatic breast cancer cells (MDA-MB-361) to study whether BKCa channel inhibition attenuates breast tumor invasion and metastasis using KCNMA1 knockdown with siRNA and biochemical inhibition with Iberiotoxin (IBTX).
Results: The Global exon array and RT-PCR showed higher KCNMA1 expression in metastatic breast cancer in brain compared to metastatic breast cancers in other organs. Our results clearly show that metastatic breast cancer cells exhibit increased BKCa channel activity, leading to greater invasiveness and transendothelial migration, both of which could be attenuated by blocking KCNMA1.
Conclusion: Determining the relative abundance of BKCa channel expression in breast cancer metastatic to brain and the mechanism of its action in brain metastasis will provide a unique opportunity to identify and differentiate between low grade breast tumors that are at high risk for metastasis from those at low risk for metastasis. This distinction would in turn allow for the appropriate and efficient application of effective treatments while sparing patients with low risk for metastasis from the toxic side effects of chemotherapy.
Figures


Similar articles
-
Role of KCNMA1 in breast cancer.PLoS One. 2012;7(8):e41664. doi: 10.1371/journal.pone.0041664. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22899999 Free PMC article.
-
Molecular interaction and functional coupling between type 3 inositol 1,4,5-trisphosphate receptor and BKCa channel stimulate breast cancer cell proliferation.Eur J Cancer. 2013 Nov;49(17):3738-51. doi: 10.1016/j.ejca.2013.07.013. Epub 2013 Aug 27. Eur J Cancer. 2013. PMID: 23992640
-
Specific expression of the human voltage-gated proton channel Hv1 in highly metastatic breast cancer cells, promotes tumor progression and metastasis.Biochem Biophys Res Commun. 2011 Aug 26;412(2):353-9. doi: 10.1016/j.bbrc.2011.07.102. Epub 2011 Jul 29. Biochem Biophys Res Commun. 2011. PMID: 21821008
-
KCNMA1-linked channelopathy.J Gen Physiol. 2019 Oct 7;151(10):1173-1189. doi: 10.1085/jgp.201912457. Epub 2019 Aug 19. J Gen Physiol. 2019. PMID: 31427379 Free PMC article. Review.
-
An emerging spectrum of variants and clinical features in KCNMA1-linked channelopathy.Channels (Austin). 2021 Dec;15(1):447-464. doi: 10.1080/19336950.2021.1938852. Channels (Austin). 2021. PMID: 34224328 Free PMC article. Review.
Cited by
-
Repositioning Trimebutine Maleate as a Cancer Treatment Targeting Ovarian Cancer Stem Cells.Cells. 2021 Apr 16;10(4):918. doi: 10.3390/cells10040918. Cells. 2021. PMID: 33923707 Free PMC article.
-
Opening large-conductance potassium channels selectively induced cell death of triple-negative breast cancer.BMC Cancer. 2020 Jun 26;20(1):595. doi: 10.1186/s12885-020-07071-1. BMC Cancer. 2020. PMID: 32586284 Free PMC article.
-
Novel insights linking BRCA1-IRIS role in mammary gland development to formation of aggressive PABCs: the case for longer breastfeeding.Am J Cancer Res. 2022 Jan 15;12(1):396-426. eCollection 2022. Am J Cancer Res. 2022. PMID: 35141026 Free PMC article.
-
A whole-genome SNP association study of NCI60 cell line panel indicates a role of Ca2+ signaling in selenium resistance.PLoS One. 2010 Sep 7;5(9):e12601. doi: 10.1371/journal.pone.0012601. PLoS One. 2010. PMID: 20830292 Free PMC article.
-
Ion channel gene expression in lung adenocarcinoma: potential role in prognosis and diagnosis.PLoS One. 2014 Jan 23;9(1):e86569. doi: 10.1371/journal.pone.0086569. eCollection 2014. PLoS One. 2014. PMID: 24466154 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

