Pyridoxal 5'-phosphate: electrophilic catalyst extraordinaire
- PMID: 19640775
- PMCID: PMC2749917
- DOI: 10.1016/j.cbpa.2009.06.023
Pyridoxal 5'-phosphate: electrophilic catalyst extraordinaire
Abstract
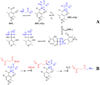
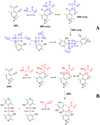
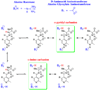
Studies of nonenzymatic electrophilic catalysis of carbon deprotonation of glycine show that pyridoxal 5'-phosphate (PLP) strongly enhances the carbon acidity of alpha-amino acids, but that this is not the overriding mechanistic imperative for cofactor catalysis. Although the fully protonated PLP-glycine iminium ion adduct exhibits an extraordinary low alpha-imino carbon acidity (pK(a)=6), the more weakly acidic zwitterionic iminium ion adduct (pK(a)=17) is selected for use in enzymatic reactions. The similar alpha-imino carbon acidities of the iminium ion adducts of glycine with 5'-deoxypyridoxal and with phenylglyoxylate show that the cofactor pyridine nitrogen plays a relatively minor role in carbanion stabilization. The 5'-phosphodianion group of PLP likely plays an important role in catalysis by providing up to 12 kcal/mol of binding energy that may be utilized for transition state stabilization.
Figures

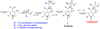

References
-
- Snell EE, Braunstein AE, Severin ES, Torchinsky YM, editors. Pyridoxal Catalysis Enzymes and Model Systems. New York: Interscience; 1968.
-
-
Barends TRM, Dunn MF, Schlichting I. Tryptophan synthase, an allosteric molecular factory. Curr.Opin.Chem.Biol. 2008;12:593–600. •• A summary of fascinating experiments that characterize the mechanism for allosteric regulation of substrate channelling at tryptophan synthase and of the reactions catalyzed by the α and β subunits.
-
-
- He X, Liu H-W. Mechanisms of enzymatic C–O bond cleavages in deoxyhexose biosynthesis. Curr.Opin.Chem.Biol. 2002;6:590–597. - PubMed
-
- Schirch V, Szebenyi DME. Serine hydroxymethyltransferase revisited. Curr.Opin.Chem.Biol. 2005;9:482–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

