Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy
- PMID: 19640900
- PMCID: PMC2775583
- DOI: 10.1152/ajprenal.00282.2009
Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy
Abstract
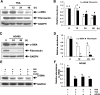
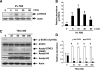
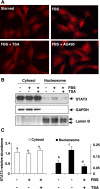
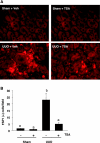
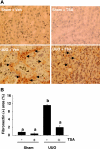
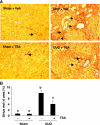
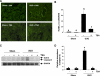
Activation of renal interstitial fibroblasts is critically involved in the development of tubulointerstitial fibrosis in chronic kidney diseases. In this study, we investigated the effect of trichostatin A (TSA), a specific histone deacetylase (HDAC) inhibitor, on the activation of renal interstitial fibroblasts in a rat renal interstitial fibroblast line (NRK-49F) and the development of renal fibrosis in a murine model of unilateral ureteral obstruction (UUO). alpha-Smooth muscle actin (alpha-SMA) and fibronectin, two hallmarks of fibroblast activation, were highly expressed in cultured NRK-49F cells, and their expression was inhibited in the presence of TSA. Similarly, administration of TSA suppressed the expression of alpha-SMA and fibronectin and attenuated the accumulation of renal interstitial fibroblasts in the kidney after the obstructive injury. Activation of renal interstitial fibroblasts was accompanied by phosphorylation of signal transducer and activator of transcription 3 (STAT3), and TSA treatment also abolished these responses. Furthermore, inhibition of the STAT3 pathway with AG490 inhibited expression of alpha-SMA and fibronectin in NRK-49F cells. Finally, TSA treatment inhibited tubular cell apoptosis and caspase-3 activation in the obstructive kidney. Collectively, we suggest that pharmacological HDAC inhibition may induce antifibrotic activity by inactivation of renal interstitial fibroblasts and inhibition of renal tubular cell death. STAT3 may mediate those actions of HDACs.
Figures
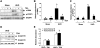
Similar articles
-
Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy.J Pharmacol Exp Ther. 2014 Aug;350(2):243-56. doi: 10.1124/jpet.113.212076. Epub 2014 May 15. J Pharmacol Exp Ther. 2014. PMID: 24833701 Free PMC article.
-
Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling.PLoS One. 2013;8(1):e54001. doi: 10.1371/journal.pone.0054001. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342059 Free PMC article.
-
A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy.Kidney Int. 2010 Aug;78(3):257-68. doi: 10.1038/ki.2010.154. Epub 2010 Jun 2. Kidney Int. 2010. PMID: 20520592
-
Application of Histone Deacetylase Inhibitors in Renal Interstitial Fibrosis.Kidney Dis (Basel). 2020 Jul;6(4):226-235. doi: 10.1159/000505295. Epub 2020 Mar 26. Kidney Dis (Basel). 2020. PMID: 32903948 Free PMC article. Review.
-
Fibroblast activation and myofibroblast generation in obstructive nephropathy.Nat Rev Nephrol. 2009 Jun;5(6):319-28. doi: 10.1038/nrneph.2009.74. Nat Rev Nephrol. 2009. PMID: 19474827 Review.
Cited by
-
Elevated expression of HDAC6 in clinical peritoneal dialysis patients and its pathogenic role on peritoneal angiogenesis.Ren Fail. 2020 Nov;42(1):890-901. doi: 10.1080/0886022X.2020.1811119. Ren Fail. 2020. PMID: 32862739 Free PMC article.
-
HDAC inhibitors in kidney development and disease.Pediatr Nephrol. 2013 Oct;28(10):1909-21. doi: 10.1007/s00467-012-2320-8. Epub 2012 Oct 7. Pediatr Nephrol. 2013. PMID: 23052657 Free PMC article. Review.
-
Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy.J Pharmacol Exp Ther. 2014 Aug;350(2):243-56. doi: 10.1124/jpet.113.212076. Epub 2014 May 15. J Pharmacol Exp Ther. 2014. PMID: 24833701 Free PMC article.
-
Single-cell multiomics reveals the complexity of TGFβ signalling to chromatin in iPSC-derived kidney organoids.Commun Biol. 2022 Nov 27;5(1):1301. doi: 10.1038/s42003-022-04264-1. Commun Biol. 2022. PMID: 36435939 Free PMC article.
-
Renoprotective effect of combined inhibition of angiotensin-converting enzyme and histone deacetylase.J Am Soc Nephrol. 2013 Apr;24(5):801-11. doi: 10.1681/ASN.2012060590. Epub 2013 Apr 4. J Am Soc Nephrol. 2013. PMID: 23559582 Free PMC article.
References
-
- Arany I, Herbert J, Herbert Z, Safirstein RL. Restoration of CREB function ameliorates cisplatin cytotoxicity in renal tubular cells. Am J Physiol Renal Physiol 294: F577–F581, 2008 - PubMed
-
- Arany I, Megyesi JK, Nelkin BD, Safirstein RL. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int 70: 669–674, 2006 - PubMed
-
- Bob FR, Gluhovschi G, Herman D, Potencz E, Gluhovschi C, Trandafirescu V, Schiller A, Petrica L, Velciov S, Bozdog G, Vernic C. Histological, immunohistochemical and biological data in assessing interstitial fibrosis in patients with chronic glomerulonephritis. Acta Histochem 110: 196–203, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

