NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse
- PMID: 19640930
- PMCID: PMC3694873
- DOI: 10.1093/cvr/cvp265
NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse
Abstract
Aims: Extracellular nucleotides are vasoactive molecules. The concentrations of these molecules are regulated by ectonucleotidases. In this study, we investigated the role of the blood vessel ectonucleotidase NTPDase1, in the vasoconstrictor effect of nucleotides using Entpd1(-/-) mice.
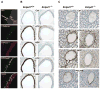
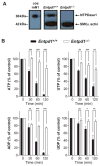
Methods and results: Immunofluorescence, enzyme histochemistry, and HPLC analysis were used to evaluate both NTPDase expression and activity in arteries and isolated vascular smooth muscle cells (VSMCs). Vascular reactivity was evaluated in vitro and mean arterial blood pressure was recorded in anesthetized mice after nucleotide i.v. infusion. Expression of nucleotide receptors in VSMCs was determined by RT-PCR. Entpd1(-/-) mice displayed a dramatic deficit of nucleotidase activity in blood vessel wall in situ and in VSMCs in comparison to control mice. In aortic rings from Entpd1(-/-) mice, UDP and UTP induced a potent and long-lasting constriction contrasting with the weak response obtained in wild-type rings. This constriction occurred through activation of P2Y(6) receptor and was independent of other uracil nucleotide-responding receptors (P2Y(2) and P2Y(4)). UDP infusion in vivo increased blood pressure and this effect was potentiated in Entpd1(-/-) mice. In addition, pressurized mesenteric arteries from Entpd1(-/-) mice displayed an enhanced myogenic response, consistent with higher local concentrations of endogenously released nucleotides. This effect was inhibited by the P2 receptor antagonist RB-2.
Conclusion: NTPDase1 is the major enzyme regulating nucleotide metabolism at the surface of VSMCs and thus contributes to the local regulation of vascular tone by nucleotides.
Conflict of interest statement
Figures





References
-
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. - PubMed
-
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. - PubMed
-
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

