Human Ubc9 contributes to production of fully infectious human immunodeficiency virus type 1 virions
- PMID: 19640976
- PMCID: PMC2753122
- DOI: 10.1128/JVI.00237-09
Human Ubc9 contributes to production of fully infectious human immunodeficiency virus type 1 virions
Abstract
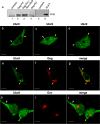
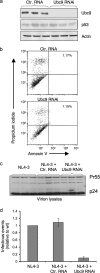
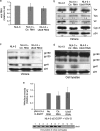
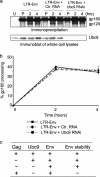
Ubc9 was identified as a cellular protein that interacts with the Gag protein of Mason-Pfizer monkey virus. We show here that Ubc9 also interacts with the human immunodeficiency virus type 1 (HIV-1) Gag protein and that their interaction is important for virus replication. Gag was found to colocalize with Ubc9 predominantly at perinuclear puncta. While cells in which Ubc9 expression was suppressed with RNA interference produced normal numbers of virions, these particles were 8- to 10-fold less infectious than those produced in the presence of Ubc9. The nature of this defect was assayed for dependence on Ubc9 during viral assembly, trafficking, and Env incorporation. The Gag-mediated assembly of virus particles and protease-mediated processing of Gag and Gag-Pol were unchanged in the absence of Ubc9. However, the stability of the cell-associated Env glycoprotein was decreased and Env incorporation into released virions was altered. Interestingly, overexpression of the Ubc9 trans-dominant-negative mutant C93A, which is a defective E2-SUMO-1 conjugase, suggests that this activity may not be required for interaction with Gag, virion assembly, or infectivity. This finding demonstrates that Ubc9 plays an important role in the production of infectious HIV-1 virions.
Figures



References
-
- Alroy, I., S. Tuvia, T. Greener, D. Gordon, H. M. Barr, D. Taglicht, R. Mandil-Levin, D. Ben-Avraham, D. Konforty, A. Nir, O. Levius, V. Bicoviski, M. Dori, S. Cohen, L. Yaar, O. Erez, O. Propheta-Meiran, M. Koskas, E. Caspi-Bachar, I. Alchanati, A. Sela-Brown, H. Moskowitz, U. Tessmer, U. Schubert, and Y. Reiss. 2005. The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production. Proc. Natl. Acad. Sci. USA 102:1478-1483. - PMC - PubMed
-
- Argasinska, J., K. Zhou, R. J. Donnelly, R. T. Hay, and C. G. Lee. 2004. A functional interaction between RHA and Ubc9, an E2-like enzyme specific for Sumo-1. J. Mol. Biol. 341:15-25. - PubMed
-
- Babic, I., E. Cherry, and D. J. Fujita. 2006. SUMO modification of Sam68 enhances its ability to repress cyclin D1 expression and inhibits its ability to induce apoptosis. Oncogene 25:4955-4964. - PubMed
-
- Batonick, M., M. Favre, M. Boge, P. Spearman, S. Honing, and M. Thali. 2005. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology 342:190-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

